Transplant recipient, immunology, medications and complications.
Transplant recipient management
Contents
- Short history of transplant
- Transplant immunology
- Donor & Recipients selection
- Mechanism of graft rejection
- Mechanism of T-cell upregulation
- Immunosuppressives
- Basic principles of Immunosuppressive regime and protocol flowsheet
- Post-transplant complications
- Overview of graft rejections
- Revised BANFF classification
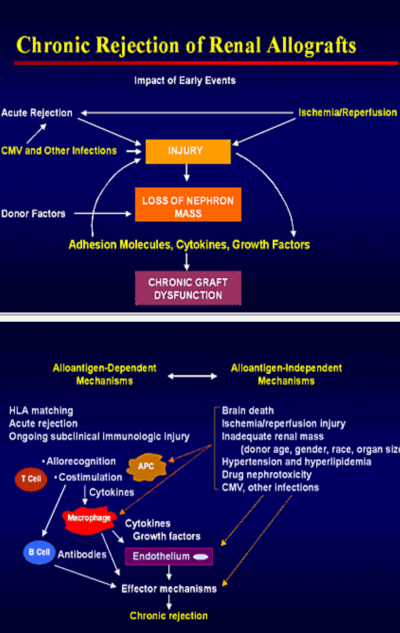
- Chronic graft rejections
- Non immune complications
__________________________________
1. Short history of transplant
- In 300 AD, Damian-Cosmas, the silverless one, did leg transplant. The success of that transplant was not known; But they were decapitated by the ruler.
- In the 1920s, vascular anastomoses developed.
- 1950s human allograft was attempted.
- Peter Medawar described cellular immunology. The close collaboration between pharmacy and clinical researchers resulted in azathioprine with prednisolone which made kidney transplantation possible in non-related individuals.
- In 1978 Cyclosporin-A became available, revolutionising transplant.
- In 1988, cellcept appeared, resistant rejections became controlled.
- Polyclonal and monoclonal antibodies revolutionised difficult graft in '90s.
- ABO incompatible transplant appeared in 2005 with advent of immunoadsorption and cascade plasmapharesis (DFPP).
- Altruistic donation appeared in 2010.
___________________________________
2. Transplant immunology & Genetics
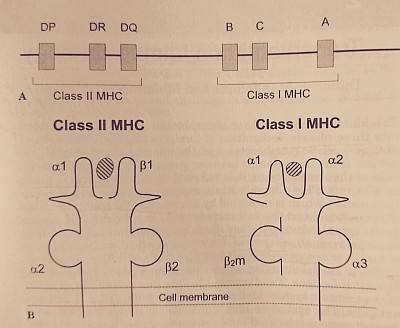
A- Major histocompatibility complex gene in loci of chromosome 6. B- Human leukocyte antigen on cell membrane binding the processed antigen.
a) Major Histocompatibility complex (MHC)
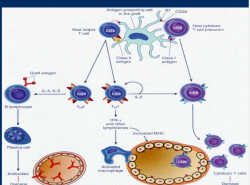
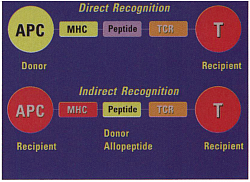
- Except for identical twin all human differ in their HLA antigen. These antigenic peptides are identified, engulfed and internalised by pinocytosis and processed, by antigen presenting cells (APC). APC presents the processed antigen to CD3 receptors of T cell. If this is associated with the CD4 receptors (CD4 positive T4 cells, T-helper cells), CD4 T-cells are activated, which in turn activate more CD4 and CD8 T-cells. If CD3 is associated with CD8 receptors (CD8 positive T suppressor cells), this results in target cells destruction.
b) Costimulatory signal
- The CD3-CD4 or CD3-CD8 receptor complex requires association of CD28 or B7 receptors, for activation of the corresponding T4 or T8 cells.
c) Graft tolerance
- If Costimulatory signal is blocked by monoclonal antibody, this results in Anergy or inactivation of corresponding T-cells resulting in graft tolerance.
d) MHC gene & family tree distribution
- HLA is determined by the MHC gene in Chomosome 6, and is of two classes.
- Class-1 is HLA - A, B & C, and present in all nucleated cells. Class-2 is HLA - DP, DQ & DR, present in T & B lymphocytes, APC & dendritic cells.
- Class-2 matching is more important in relation to the graft rejection.
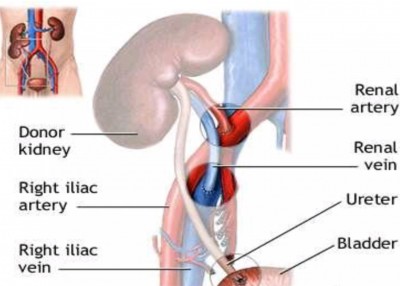
3. Donor & recipient selection
LRD, S to S, & DD
Corresponding recipients to be evaluated according to the standard criterion of fitness :
Immunological, surgical, urological, cardiological, serological, virological, neoplastic, ethical, psychological, behavioral aspects.
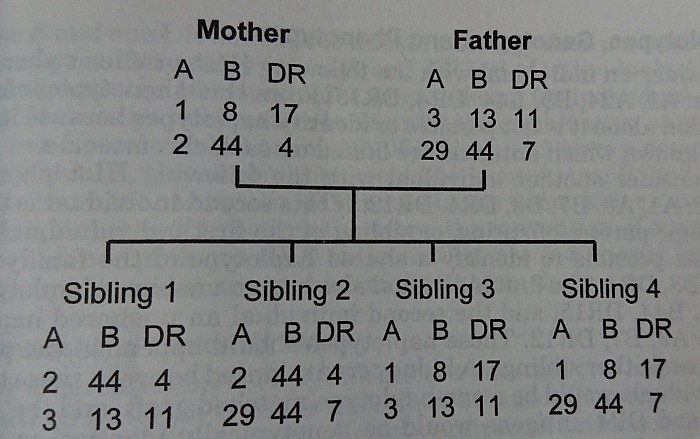
Tissue type, match, and sensitization.
# ABO-Competible & ABO-Incompetible
# HLA antigen typing, 0 to 6 antigen match
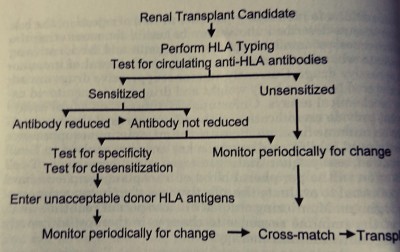
# Sensitization status by Panel reactive antibody (PRA) estimate
# In case of selected donor, donor specific antibody (DSA) testing in recipient, direct crossmatch (xm) - b cell and t cell xm, DTT treated xm, flow cytometry xm.
Desensitization
# ABO-i: DFPP or Immunoadsorption, till ABO antibody titer drops to 1/8-1/16 with IV Rituximab per protocol.
# Direct Cross-match positive (T or B cell): DFPP 4 to 5 times till cross-match becomes negative, with IV Thymoglobulin and IV CMV immunoglobulin after each DFPP according to protocol.
# High PRA (>25%): IV thymoglobulin induction for first 10 days with FK506, MMF & prednisolone as per protocol.
# DSA (Donor specific antibody) positive: IV Rituximab with iv IgG and DFPP per protocol.
___________________________________
4. Mechanism of graft rejection
Recipient and donor selection
Antigen presentation by Antigen presenting cell (APC) to CD4/ CD8. CD4 activation, CD8 cytotoxicity, subsequent inflammation & cell destruction.
T-cell activation: APC presents ag to t-cell reception (TCR CD3), If associated with CD4, T4(help t-cell) is activated. If CD8 is associated T8 cytotoxic t-cell is activated. When cd28 attached to B7 of APC, called costimulation. Tcell activation occur
Allorecognition
___________________________________
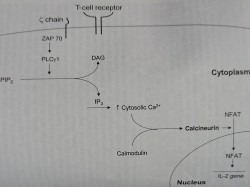
5. Mechanism of T-cell upregulation
Mechanism of action of immunosuppressive agents are basically inhibition of T cell activation.
This can be seen at three signals.
Signal 1
The calcium-dependent signal induced by TCR stimulation results in calcineurin activation, a process inhibited by cyclosporine and tacrolimus.
Calcineurin dephosphorylates NFAT (nuclear factor of activated T cells), enabling it to enter the nucleus and bind to the IL-2 promoter.
Signal 2
Costimulatory signals are necessary to optimize T cell IL-2 gene transcription, prevent T cell anergy, and inhibit T cell apoptosis.
Experimental agents, but not current immunosuppressive agents, interrupt these intracellular signals. Inhibition of Co-stimulatory signals induce t-cell anergy. This induces tolerance.
Signal 3
IL-2 receptor stimulation induces the cell to enter the cell cycle and proliferate. Signal 3 may be blocked by IL-2 receptor-Ab or by sirolimus, which inhibits mTOR kinase activation.
Following progression into the cell cycle, azathioprine and MMF interrupt DNA replication by inhibiting purine synthesis.
___________________________________
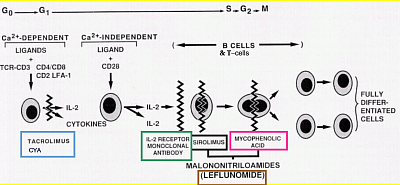
6. Immunosuppressives (LINK)
Corticosteroids
Corticosteroids bind to cytoplasmic receptors, enter the nucleus, and inhibit cytokine gene transcription in both T cells and the APCs. Corticosteroids also inhibit nuclear factor of activated T cells activation (NFAT).
CNI
Unfortunately, Cyclosporin and Tacrolimus have major side effects on Nephrotoxic it. Acute CNI nephrotoxicity is reversible, but chronic CNI toxicity leads to kidney damage with pathological striped fibrosis. Acute toxicity is mainly due to CNI induced vasoconstriction, and also slows recovery from post operative ATN. It potentiates nephrotoxicity due to other substances. Other acute toxicities are tremor, palmar and plantar paresthesia, hyperglycemia, hepatotoxicity, hypertrichosis, gingival hypertrophy, and hyperkalemia.
These are less frequent with tacrolimus.
___________________________________
7. Transplantation protocol and Basic principles of Immunosuppressive regimen
Pre/per/post-op immunosuppressive regimen
1. Desensitization
2. Preoperative - desensitization
3. Peroperative - induction
4. Postoperative - maintenance and treatment for rejection episodes.
5. Prophylaxis - CMV, PCP & mycosis.
6. Monitoring
1). Desensitization
In LRD transplant: described above in recepient selection. Subsequent to that, in general, you need to start CNI, MMF at -d3 in LRD cases.
In DD transplant: the induction depending on PRA (thymoglobulin in high PRA) & cross match - B or T cell, (thymoglobulin x5-10 doses and DFPP).
In DSA (Donor specific antibody) positive recipient: IV Rituximab with iv IgG and DFPP.
2) Induction
- IV Basiliximab 20 mg x2 at 4 days interval and IV MP 500 mg x2 doses, followed by CNI + MMF + Prednisolone.
- In cases of ATN or primary graft non-function, Give CNI holiday, and use thymoglobulin induction as above, with MMF & prednisone. Followed by CNI or. mTOR inhibitors with MMF+prednisone.
3) Immunosuppressive induction protocols
4) Maintenance
CNI/mTOR + AZA /MMF + Prednisolone. Depends on PRA, Crossmatch, retransplant, CNI toxicity, malignancy, viremia, proteinuria etc.
CNI reduced dose protocol
Follow Transfom(TX) study, mTOR with half trough level CNI with Steroid.
CNI free protocol/ Steroid free
Follow 3C study, IV CAMPATH (20mgX2 at 3 months gap) with mTOR +MMF or FK506 +MMF.
5) Prophylaxis
All recipients need Bactrim and valgancyclovir for first 3 months. For others as follows:
- For FK506 /MMF, longterm Bactrim.
- For CMV d+r-, longterm valgancyclovir.
- For BKV, reduced CNI /MMF.
6) Monitoring
- CVP, BP, Urine output, drain output,.
- Graft function, drug levels, sepsis & blood counts, ufeme & UPCR, lipid, & fasting glucose, HBA1C.
- Graft perfusion by DTPA-T99 renal scan, renal doppler.
- US graft & native kidneys.
- Virology for CMV, BKV, HCV, HBV, EBV/Pvb19 as indicated.
- Monitoring for malignancy/PTLD
7). Adjuvant therapy
- IV Furosemide - post transplant days with reduced urine output with and CVP >12, you can give, anticipating some degree of prerenal AKI.
- Nifedipine LA: CNI is a known antagonize Vado constriction specially transplanted renal artary. Nifedipine is known to antagonise it. useful at the post operative time.
- IV Domaine @2mcg/hour: anacdotally used by some centres as a Renal artary dilator for increasing renal perfusion.
- Diltiazem: as a C450 enzyme inhibitor, reduces FK and mTOR dosages.
Vaccinations
- Pretransplant- HBV, VZV, Fluvax, N. Meningitis, pneumococcal.
- Posttransplant - fluvax, pneumococcal, N. Meningitis, HBV.
__________________________________
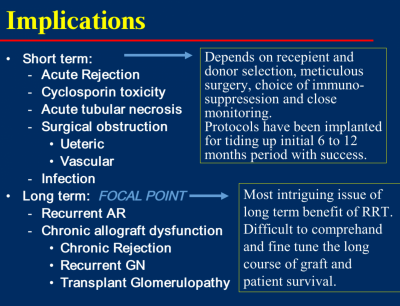
8. Post transplant complications
Implications of renal transplantation
Overview of graft rejection
2023 BANFF CLASSIFICATION
- Antibody-mediated rejection (ABMR) and
- T cell–mediated rejection (TCMR)
- Borderline changes
Active ABMR:
All 3 criteria must be met for diagnosis:1. Histologic evidence of acute tissue injury, including one or more of the following: a) Microvascular inflammation (g > 0 and/or ptc > 0), in the absence of recurrent or de novo glomerulonephritis, although in the presence of acute TCMR, borderline infiltrate, or infection, ptc ≥ 1 alone is not sufficient and g must be ≥ 1, b) Intimal or transmural arteritis (v > 0), c) Acute thrombotic microangiopathy, in the absence of any other cause, d) Acute tubular injury, in the absence of any other apparent cause.
2. Evidence of current/recent antibody interaction with vascular endothelium, including 1 or more of the following: a) Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by IF on frozen sections, or C4d > 0 by IHC on paraffin sections). b) At least moderate microvascular inflammation ([g + ptc] ≥2) in the absence of recurrent or de novo glomerulonephritis, although in the presence of acute TCMR, borderline infiltrate, or infection, ptc ≥ 2 alone is not sufficient and g must be ≥1. Increased expression of gene transcripts/classifiers in the biopsy tissue strongly associated with ABMR, if thoroughly validated.
3. Serologic evidence of donor-specific antibodies (DSA to HLA or other antigens). C4d staining or expression of validated transcripts/classifiers as noted above in criterion 2 may substitute for DSA; however thorough DSA testing, including testing for non-HLA antibodies if HLA antibody testing is negative, is strongly advised whenever criteria 1 and 2 are met.
Chronic active ABMR
All 3 criteria must be met for diagnosis²
- Morphologic evidence of chronic tissue injury, including 1 or more of the following:
- Transplant glomerulopathy (cg >0) if no evidence of chronic TMA or chronic recurrent/de novo glomerulonephritis; includes changes evident by electron microscopy (EM) alone (cg1a)
- Severe peritubular capillary basement membrane multilayering (requires EM). (Arterial intimal fibrosis of new onset, excluding other causes; leukocytes within the sclerotic intima favor chronic ABMR if there is no prior history of TCMR, but are not required).
C4d Staining without Evidence of Rejection; all 4 features must be present for Diagnosis. Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by IF on frozen sections, or C4d>0 by IHC on paraffin sections)
Criterion 1 for active or chronic, active ABMR not met
No molecular evidence for ABMR as in criterion 2 for active and chronic, active Abmr.
No acute or chronic active TCMR, or borderline changes.
Borderline changes
Suspicious (Borderline) for acute TCMR.
Foci of tubulitis (t > 0) with minor interstitial inflammation (i0 or i1), or moderate-severe interstitial inflammation (i2 or i3) with mild (t1) tubulitis; retaining the i1 threshold for borderline with t > 0 is permitted although this must be made transparent in reports and publications
No intimal or transmural arteritis (v = 0).
Acute TCMR
- Grade IA Interstitial inflammation involving >25% of nonsclerotic cortical parenchyma (i2 or i3) with moderate tubulitis (t2) involving 1 or more tubules, not including tubules that are severely atrophic.
- Grade IB Interstitial inflammation involving >25% of nonsclerotic cortical parenchyma (i2 or i3) with severe tubulitis (t3) involving 1 or more tubules, not including tubules that are severely atrophic.
- Grade IIA 1 Mild to moderate intimal arteritis (v1), with or without interstitial inflammation and/or tubulitis.
- Grade IIB1 Severe intimal arteritis (v2), with or without interstitial inflammation
and/or tubulitis.
- Grade III1 Transmural arteritis and/or arterial fibrinoid necrosis of medial smooth muscle with accompanying mononuclear cell intimal arteritis (v3), with or without interstitial inflammation and/or tubulitis.
- 2023 BANFF CLASSIFICATION
Chronic Graft rejection
Chronic Active TCMR
- Grade IA Interstitial inflammation involving >25% of the total cortex (ti score 2 or3) and >25% of the sclerotic cortical parenchyma (i-IFTA score 2 or 3) with moderate tubulitis (t2) involving 1 or more tubules, not including severely atrophic tubules; other known causes of i-IFTA should be ruled out.
- Grade IB Interstitial inflammation involving >25% of the total cortex (ti score 2 or 3) and >25% of the sclerotic cortical parenchyma (i-IFTA score 2 or 3) with severe tubulitis (t3) involving 1 or more tubules, not including severely atrophic tubules; other known causes of i-IFTA should be ruled out.
- Grade II Chronic allograft arteriopathy (arterial intimal fibrosis with mononuclear cell inflammation in fibrosis and formation of neointima).
It should be noted that these arterial lesions may be indicative of ABMR, TCMR, or mixed ABMR/TCMR. “v” lesions and chronic allograft arteriopathy are only scored in arteries having a continuous media with ≥2 smooth muscle layers.
Lesions of chronic active ABMR can range from primarily active lesions with early transplant glomerulopathy (TG) evident only by EM (cg1a) to those with advanced TG and other chronic changes in addition to active microvascular inflammation. For biopsy specimens showing TG and/or peritubular capillary basement membrane multilayering in the absence of evidence of current/recent antibody interaction with the endothelium (criterion 2) but with a prior documented diagnosis of active or chronic active ABMR or documented prior evidence of DSA, the term “chronic ABMR” should be applied.
Indicates ≥7 layers in 1 cortical peritubular capillary and ≥5 in 2 additional capillaries, avoiding portions cut tangentially. The clinical significance of these findings may be quite different in grafts exposed to anti–blood group antibodies (ABO-incompatible allografts), where they do not appear to be injurious to the graft and may represent accommodation. However, with anti-HLA antibodies, such lesions may progress to chronic ABMR, and more outcome data are needed.
A severely atrophic tubule is defined as one with each of the following 3 features: a diameter <25% of that of unaffected or minimally affected tubules on the biopsy, an undifferentiated-appearing, cuboidal or flattened epithelium, and pronounced wrinkling and/or thickening of the tubular basement membrane.