STUDIES AND TRIALS IN CARDIOLOGY
ANTI-PLATELET TRIALS 1. TARDIS TRIAL 2. Dual Anti-platelet trial
1. TARDIS Trial: Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Prof Philip M Bath, FMedSci
Among patients with recent cerebral ischaemia, intensive antiplatelet therapy did not reduce the incidence and severity of recurrent stroke or TIA, but did significantly increase the risk of major bleeding. Triple antiplatelet therapy should not be used in routine clinical practice.
2. Dual Antiplatelet Therapy
First, prolonged DAPT bleeding risk, and we had a trial of prolonged DAPT. Shorter durations of DAPT are possible, particularly in high-bleeding-risk patients.
Second, notion of going from DAPT to single antiplatelet therapy.
Third, de-escalation. to Aspirin or with Clopidogrel or Ticagrelor.
Fourth, Antiplatelet anticoagulant
Old time: Dextrin, Coumadin, Ticlid, or Persantine. High chance to bleed, and in fact, they did bleed and then stent thrombosis.
Currently revolutionized with DAPT.
After an ACS event, you would need a year. Obviously, then, people began to say more than a year was needed. We had PEGASUS and the DAPT study to see if we needed to extend beyond it. Rightright dose of aspirin? by ADAPTABLE study for dose of aspirin.
2.PEGASUS-TIMI: Long-Term Use of Ticagrelor in Patients with Prior Myocardial InfarctionList of authors. Marc P. Bonaca, etal.
BACKGROUND
The potential benefit of dual antiplatelet therapy beyond 1 year after a myocardial infarction has not been established. We investigated the efficacy and safety of ticagrelor, a P2Y12 receptor antagonist with established efficacy after an acute coronary syndrome, in this context.
METHODS
We randomly assigned, in a double-blind 1:1:1 fashion, 21,162 patients who had had a myocardial infarction 1 to 3 years earlier to ticagrelor at a dose of 90 mg twice daily, ticagrelor at a dose of 60 mg twice daily, or placebo. All the patients were to receive low-dose aspirin and were followed for a median of 33 months. The primary efficacy end point was the composite of cardiovascular death, myocardial infarction, or stroke. The primary safety end point was Thrombolysis in Myocardial Infarction (TIMI) major bleeding.
RESULTS
The two ticagrelor doses each reduced, as compared with placebo, the rate of the primary efficacy end point, with Kaplan–Meier rates at 3 years of 7.85% in the group that received 90 mg of ticagrelor twice daily, 7.77% in the group that received 60 mg of ticagrelor twice daily, and 9.04% in the placebo group (hazard ratio for 90 mg of ticagrelor vs. placebo, 0.85; 95% confidence interval [CI], 0.75 to 0.96; P=0.008; hazard ratio for 60 mg of ticagrelor vs. placebo, 0.84; 95% CI, 0.74 to 0.95; P=0.004). Rates of TIMI major bleeding were higher with ticagrelor (2.60% with 90 mg and 2.30% with 60 mg) than with placebo (1.06%) (P<0.001 for each dose vs. placebo); the rates of intracranial hemorrhage or fatal bleeding in the three groups were 0.63%, 0.71%, and 0.60%, respectively.
CONCLUSIONS
In patients with a myocardial infarction more than 1 year previously, treatment with ticagrelor significantly reduced the risk of cardiovascular death, myocardial infarction, or stroke and increased the risk of major bleeding.
3. DAPT Study (Longer duration): Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting StentsList of authors. Robert W, et al.
BACKGROUND
Dual antiplatelet therapy is recommended after coronary stenting to prevent thrombotic complications, yet the benefits and risks of treatment beyond 1 year are uncertain.
METHODS
Patients were enrolled after they had undergone a coronary stent procedure in which a drug-eluting stent was placed. After 12 months of treatment with a thienopyridine drug (clopidogrel or prasugrel) and aspirin, patients were randomly assigned to continue receiving thienopyridine treatment or to receive placebo for another 18 months; all patients continued receiving aspirin. The coprimary efficacy end points were stent thrombosis and major adverse cardiovascular and cerebrovascular events (a composite of death, myocardial infarction, or stroke) during the period from 12 to 30 months. The primary safety end point was moderate or severe bleeding.
RESULTS
A total of 9961 patients were randomly assigned to continue thienopyridine treatment or to receive placebo. Continued treatment with thienopyridine, as compared with placebo, reduced the rates of stent thrombosis (0.4% vs. 1.4%; hazard ratio, 0.29 [95% confidence interval {CI}, 0.17 to 0.48]; P<0.001) and major adverse cardiovascular and cerebrovascular events (4.3% vs. 5.9%; hazard ratio, 0.71 [95% CI, 0.59 to 0.85]; P<0.001). The rate of myocardial infarction was lower with thienopyridine treatment than with placebo (2.1% vs. 4.1%; hazard ratio, 0.47; P<0.001). The rate of death from any cause was 2.0% in the group that continued thienopyridine therapy and 1.5% in the placebo group (hazard ratio, 1.36 [95% CI, 1.00 to 1.85]; P=0.05). The rate of moderate or severe bleeding was increased with continued thienopyridine treatment (2.5% vs. 1.6%, P=0.001). An elevated risk of stent thrombosis and myocardial infarction was observed in both groups during the 3 months after discontinuation of thienopyridine treatment.
CONCLUSIONS
Dual antiplatelet therapy beyond 1 year after placement of a drug-eluting stent, as compared with aspirin therapy alone, significantly reduced the risks of stent thrombosis and major adverse cardiovascular and cerebrovascular events but was associated with an increased risk of bleeding.
4. Additional study no benefit with longer DAPT.
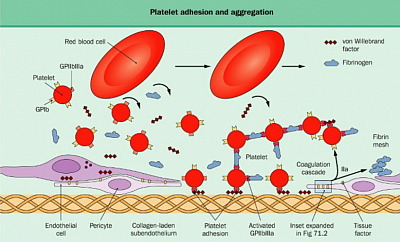
Cardiovascular and cerebrovascular events commonly arise from atherosclerotic plaque rupture that produces platelet activation, thrombus formation, and reduction of blood flow to the brain or heart. The inhibition of platelets with aspirin is effective in the secondary prevention of acute coronary events.1 The addition of clopidogrel (i.e., dual antiplatelet therapy), a platelet P2Y12-receptor antagonist, produces even greater secondary prevention of coronary events in high-risk patients for up to 1 year.2 Second-generation P2Y12 inhibitors (i.e., prasugrel and ticagrelor) produce further reductions in the risk of ischemic events over the same time frame, albeit with more bleeding complications.3,4
Dual antiplatelet therapy is recommended for 1 year after an acute coronary syndrome, but the effect of longer-term therapy is not clear. Concern exists regarding the balance between reducing the risk of cardiovascular events and the risk of bleeding complications, because bleeding complications are linked to adverse outcomes in patients with an acute coronary syndrome.5 Bonaca et al., in the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared with Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial,6 provide insight into this balance in high-risk patients with a previous myocardial infarction. In their study, the results of which are now reported in the Journal, they randomly assigned 21,162 patients to placebo or ticagrelor. Because long-term P2Y12 inhibition increases bleeding risk, the investigators compared two doses of ticagrelor (60 mg and 90 mg) to maximize information derived from the trial concerning drug efficacy versus adverse events. As compared with placebo, either dose of ticagrelor was associated with a 15% decrease in the rate of the primary end point of death from cardiovascular causes, myocardial infarction, or stroke. However, ticagrelor treatment also increased clinically significant bleeding complications by a factor of 2.3 to 2.7 and transfusions by a factor of 3.1 to 3.8. There was similar efficacy in the reduction of the rate of the primary end point with either ticagrelor dose, suggesting that the lower dose should be preferred in this patient population because it may limit clinically significant bleeding events.
5. EVOLVE study: (Shorter duration of DAPT) . Evaluation of 3-Month Dual Antiplatelet Therapy in High Bleeding Risk Patients Treated With a Bioabsorbable Polymer-Coated Everolimus-Eluting Stent. Robert Stoler, et al.
Background:
Prolonged dual antiplatelet therapy (DAPT) after percutaneous coronary intervention is associated with increased bleeding, despite a reduced incidence of ischemic events. The SYNERGY everolimus-eluting stent is a thin-strut platinum-chromium stent that elutes everolimus from a thin abluminal layer of bioabsorbable polymer. These design elements may facilitate rapid endothelialization and enable shorter-duration DAPT.
Methods:
EVOLVE Short DAPT prospectively evaluated the safety of 3-month DAPT in high bleeding risk patients treated with the SYNERGY everolimus-eluting stent, enrolling 2009 patients at 110 global sites. Patients with acute myocardial infarction or complex lesions were excluded. After percutaneous coronary intervention, patients were required to take DAPT (aspirin+P2Y12 inhibitor) for 3 months, except those on chronic anticoagulation in whom aspirin was optional. Patients free of events (stroke, myocardial infarction, revascularization, and stent thrombosis) who discontinued P2Y12 inhibitor at 3 months, but continued aspirin, and had at least 1 year of follow-up or an end point event were included in the primary analysis. Two powered coprimary end points were (1) death/myocardial infarction compared with a historical control and (2) study stent-related definite/probable stent thrombosis compared to a performance goal.
Results:
The analysis population consisted of 1487 patients. The adjusted rate of death/myocardial infarction between 3 and 15 months was 5.6% among patients receiving 3-month DAPT versus 5.7% patients in the 12-month DAPT control (propensity adjusted difference=−0.12%; 97.5% upper bound=1.63% which was less than the prespecified margin of 2.52; Pnon-inferiority=0.0016). The rate of study stent-related stent thrombosis between 3-15 months was 0.2% in the 3-month DAPT group (97.5% upper bound=0.63%; P=0.0005 for comparison to 1% performance goal).
Conclusions:
Favorable rates of ischemic outcomes were observed among selected high bleeding risk patients undergoing percutaneous coronary intervention with the SYNERGY everolimus-eluting stent who tolerated 3 months of P2Y12 inhibitor and then discontinued it, supporting the safety of abbreviated DAPT with this stent platform.
INTERVENTIONAL CARDIOLOGY
Interventional Cardiology and Cardiac Surgery
FFR/PCI
Complete revascularization using FFR guiding vs bypass surgery, what will the outcomes be? Better with CABG with advanced disease. However, FFR, in a less severe range, it is very helpful.
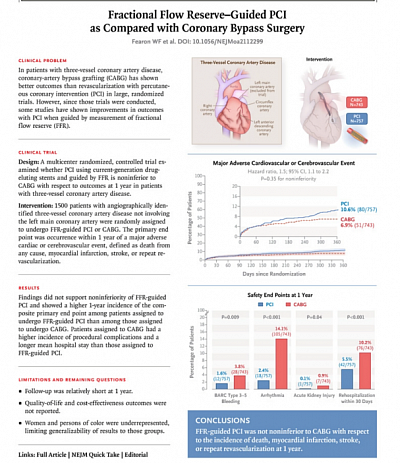
Fractional Flow Reserve–Guided PCI as Compared with Coronary Bypass Surgery. William F. Fearon, January 13, 2022, N Engl J Med 2022
Abstract: Noninferiority trial, patients with three-vessel coronary artery disease were randomly assigned to undergo CABG or FFR-guided PCI with current-generation zotarolimus-eluting stents. The primary end point was the occurrence within 1 year of a major adverse cardiac or cerebrovascular event, defined as death from any cause, myocardial infarction, stroke, or repeat revascularization. Noninferiority of FFR-guided PCI to CABG was prespecified as an upper boundary of less than 1.65 for the 95% confidence interval of the hazard ratio. Secondary end points included a composite of death, myocardial infarction, or stroke; safety was also assessed.
Results & Conclusions: A total of 1500 patients. In patients with three-vessel coronary artery disease, FFR-guided PCI was not found to be noninferior to CABG with respect to the incidence of a composite of death, myocardial infarction, stroke, or repeat revascularization at 1 year.
----------------
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design (AHJ 2018)
Background: Prior trials comparing a strategy of optimal medical therapy with or without revascularization have not shown that revascularization reduces cardiovascular events in patients with stable ischemic heart disease (SIHD). However, those trials only included participants in whom coronary anatomy was known prior to randomization and did not include sufficient numbers of participants with significant ischemia. It remains unknown whether a routine invasive approach offers incremental value over a conservative approach with catheterization reserved for failure of medical therapy in patients with moderate or severe ischemia.
Methods: The ISCHEMIA trial is a National Heart, Lung, and Blood Institute supported trial, designed to compare an initial invasive or conservative treatment strategy for managing SIHD patients with moderate or severe ischemia on stress testing. Five thousand one-hundred seventy-nine participants have been randomized. Key exclusion criteria included estimated glomerular filtration rate (eGFR) <30 mL/min, recent myocardial infarction (MI), left ventricular ejection fraction <35%, left main stenosis >50%, or unacceptable angina at baseline. Most enrolled participants with normal renal function first underwent blinded coronary computed tomography angiography (CCTA) to exclude those with left main coronary artery disease (CAD) and without obstructive CAD. All randomized participants receive secondary prevention that includes lifestyle advice and pharmacologic interventions referred to as optimal medical therapy (OMT). Participants randomized to the invasive strategy underwent routine cardiac catheterization followed by revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery, when feasible, as selected by the local Heart Team to achieve optimal revascularization. Participants randomized to the conservative strategy undergo cardiac catheterization only for failure of OMT. The primary endpoint is a composite of cardiovascular (CV) death, nonfatal myocardial infarction (MI), hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest. Assuming the primary endpoint will occur in 16% of the conservative group within 4 years, estimated power exceeds 80% to detect an 18.5% reduction in the primary endpoint. Major secondary endpoints include the composite of CV death and nonfatal MI, net clinical benefit (primary and secondary endpoints combined with stroke), angina-related symptoms and disease-specific quality of life, as well as a cost-effectiveness assessment in North American participants. Ancillary studies of patients with advanced chronic kidney disease and those with documented ischemia and non-obstructive coronary artery disease are being conducted concurrently.
Conclusions: ISCHEMIA will provide new scientific evidence regarding whether an invasive management strategy improves clinical outcomes when added to optimal medical therapy in patients with SIHD and moderate or severe ischemia.
_________
LAAOS III, the left atrial appendage occlusion trial
well designed, well powered.
Closing off that left atrial appendage at the time of surgery reduced the risk for embolic events by one third, as I recall. I think it is important. I think it probably should change practice, but ~then it brings up the question of left atrial appendage closure percutaneously.~
Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. Richard P. Whitlock. June 3, 2021, N Engl J Med 2021; 384
Abstract: Surgical occlusion of the left atrial appendage has been hypothesized to prevent ischemic stroke in patients with atrial fibrillation. Multicenter, randomized trial involving participants with atrial fibrillation and a CHA2DS2-VASc score of at least 2 (on a scale from 0 to 9, with higher scores indicating greater risk of stroke) who were scheduled to undergo cardiac surgery for another indication. The participants were randomly assigned to undergo or not undergo occlusion of the left atrial appendage during surgery; all the participants were expected to receive usual care, including oral anticoagulation, during follow-up. The primary outcome was the occurrence of ischemic stroke (including transient ischemic attack with positive neuroimaging) or systemic embolism.
The participants were followed for a mean of 3.8 years. A total of 92.1% of the participants received the assigned procedure, and at 3 years, 76.8% of the participants continued to receive oral anticoagulation.
Result & Conclusion: Stroke or systemic embolism occurred in 114 participants (4.8%) in the occlusion group and in 168 (7.0%) in the no-occlusion group (hazard ratio, 0.67; 95% confidence interval, 0.53 to 0.85; P=0.001). The incidence of perioperative bleeding, heart failure, or death did not differ significantly between the trial groups.
3. Cardiac Electrophysiology Studies
Acronyms of the trials
ADEPT
ADvanced Elements of Pacing Randomized Controlled Trial
Atrial Dynamic Overdrive Pacing Trial
Atrial Overdrive Pacing Study
Ablate and Pace in Atrial Fibrillation
ASymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial
ATTEST
ATrial Therapy Efficacy and Safety Trial
AV Node Ablation with CLS and CRT Pacing Therapies for Treatment of AF trial
Bradycardia detection in Bundle Branch Block
Bi vs. Left Ventricular Pacing: an International Pilot Evaluation on Heart Failure Patients with Ventricular Arrhythmias
Biventricular pacing for atrioventricular block to prevent cardiac desynchronization
Biventricular versus right ventricular pacing in patients with AV block
Biventricular versus LEFT Univentricular Pacing with ICD Back-up in Heart Failure Patients
CArdiac REsynchronization in Heart Failure
CABANA
Catheter Ablation versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial.
CAST I and II
Cardiac Arrhythmia Survival Trial (CAST I and II) data. Association between ease of suppression of ventricular arrhythmia and survival. Goldstein et al. Circulation. 1995
CASTLE-AF
Catheter Ablation versus Standard Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) Trial.
CLinical Evaluation on Advanced Resynchronization
COnventional vs. Biventricular Pacing in Heart Failure and Bradyarrhythmia
COmparison of Medical Therapy, Pacing and Defibrillation in Heart Failure
DANPACE
DANish Multicenter Randomized Trial on Single Lead Atrial PACing vs. Dual Chamber Pacing in Sick Sinus Syndrome
The Device Evaluation of CONTAK RENEWAL 2 and EASYTRAK 2: Assessment of Safety and Effectiveness in Heart Failure
Optimization Study Using the QuickOpt Method
Evaluation of Resynchronization Therapy for Heart Failure in Patients with a QRS Duration GREATER Than 120 ms
Evaluation of Resynchronization Therapy for Heart Failure in Patients with a QRS Duration Lower Than 120 ms
HOmburg BIventricular PACing Evaluation
Italian Network on Congestive Heart Failure
International retrospective study
Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group studyRoderick Tung et al. Heart Rhythm. 2015
ISSUE
International Study on Syncope of Unexplained Etiology
Multicenter Automatic Defibrillator Trial
Multicenter InSync RAndomized CLinical Evaluation
MOde Selection Trial in Sinus-Node Dysfunction
MUltisite STimulation In Cardiomyopathies
Optimal Pacing SITE
Pacing to Avoid Cardiac Enlargement
Left Ventricular-Based Cardiac Stimulation Post AV Nodal Ablation Evaluation
PAcing THerapies in Congestive Heart Failure II Study Group
Pacing In Prevention of Atrial Fibrillation Study
Prevention of Immediate Reinitiation of Atrial Tachyarrhythmias
Prevention Or Termination Study
PREventing VENTricular Dysfunction in Pacemaker Patients Without Advanced Heart Failure
PRedictors Of Response to Cardiac Resynchronization Therapy
Resynchronization–Defibrillation for Ambulatory Heart Failure Trial
RethinQ
Cardiac REsynchronization THerapy IN Patients with Heart Failure and Narrow QRS
REVERSE
REsynchronization reVErses Remodelling in Systolic left vEntricular dysfunction
Study of Atrial Fibrillation Reduction
Sudden Cardiac Death in Heart Failure Trial
The SMARTDelay Determined AV Optimization: a Comparison with Other AV Delay Methods Used in Cardiac Resynchronization Therapy
STAR AF II
Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF II). NEJM 2015
Background: Catheter ablation is less successful for persistent atrial fibrillation than for paroxysmal atrial fibrillation. Guidelines suggest that adjuvant substrate modification in addition to pulmonary-vein isolation is required in persistent atrial fibrillation.
Methods: We randomly assigned 589 patients with persistent atrial fibrillation in a 1:4:4 ratio to ablation with pulmonary-vein isolation alone (67 patients), pulmonary-vein isolation plus ablation of electrograms showing complex fractionated activity (263 patients), or pulmonary-vein isolation plus additional linear ablation across the left atrial roof and mitral valve isthmus (259 patients). The duration of follow-up was 18 months. The primary end point was freedom from any documented recurrence of atrial fibrillation lasting longer than 30 seconds after a single ablation procedure.
Results: Procedure time was significantly shorter for pulmonary-vein isolation alone than for the other two procedures (P<0.001). After 18 months, 59% of patients assigned to pulmonary-vein isolation alone were free from recurrent atrial fibrillation, as compared with 49% of patients assigned to pulmonary-vein isolation plus complex electrogram ablation and 46% of patients assigned to pulmonary-vein isolation plus linear ablation (P=0.15). There were also no significant differences among the three groups for the secondary end points, including freedom from atrial fibrillation after two ablation procedures and freedom from any atrial arrhythmia. Complications included tamponade (three patients), stroke or transient ischemic attack (three patients), and atrioesophageal fistula (one patient).
Conclusions: Among patients with persistent atrial fibrillation, we found no reduction in the rate of recurrent atrial fibrillation when either linear ablation or ablation of complex fractionated electrograms was performed in addition to pulmonary-vein isolation. (
The SYncope DIagnosis and Treatment
Vasovagal SYNcope and PACing
TARgeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy
Effects of Oral THEOphylline and of Permanent PACEmaker on the Symptoms and Complications of Sick Sinus Syndrome
VAsovagal Syncope International Study on PaceMaker therapy
Vasodilator in HEart Failure Trial
Second Vasovagal Pacemaker Study (VPS II)
VTACH
Catheter Ablation of Stable Ventricular Tachycardia before Defibrillator Implantation in Patients with Coronary Heart Disease (VTACH) trial
--------------------
Some trials details
CABANA
Importance: Catheter ablation is effective in restoring sinus rhythm in atrial fibrillation (AF), but its effects on long-term mortality and stroke risk are uncertain.
Objective: To determine whether catheter ablation is more effective than conventional medical therapy for improving outcomes in AF.
Design, setting, and participants: The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation trial is an investigator-initiated, open-label, multicenter, randomized trial involving 126 centers in 10 countries. A total of 2204 symptomatic patients with AF aged 65 years and older or younger than 65 years with 1 or more risk factors for stroke were enrolled from November 2009 to April 2016, with follow-up through December 31, 2017.
Interventions: The catheter ablation group (n = 1108) underwent pulmonary vein isolation, with additional ablative procedures at the discretion of site investigators. The drug therapy group (n = 1096) received standard rhythm and/or rate control drugs guided by contemporaneous guidelines.
Main outcomes and measures: The primary end point was a composite of death, disabling stroke, serious bleeding, or cardiac arrest. Among 13 prespecified secondary end points, 3 are included in this report: all-cause mortality; total mortality or cardiovascular hospitalization; and AF recurrence.
Results: Of the 2204 patients randomized (median age, 68 years; 37.2% female; 42.9% had paroxysmal AF and 57.1% had persistent AF), 89.3% completed the trial. Of the patients assigned to catheter ablation, 1006 (90.8%) underwent the procedure. Of the patients assigned to drug therapy, 301 (27.5%) ultimately received catheter ablation. In the intention-to-treat analysis, over a median follow-up of 48.5 months, the primary end point occurred in 8.0% (n = 89) of patients in the ablation group vs 9.2% (n = 101) of patients in the drug therapy group (hazard ratio [HR], 0.86 [95% CI, 0.65-1.15]; P = .30). Among the secondary end points, outcomes in the ablation group vs the drug therapy group, respectively, were 5.2% vs 6.1% for all-cause mortality (HR, 0.85 [95% CI, 0.60-1.21]; P = .38), 51.7% vs 58.1% for death or cardiovascular hospitalization (HR, 0.83 [95% CI, 0.74-0.93]; P = .001), and 49.9% vs 69.5% for AF recurrence (HR, 0.52 [95% CI, 0.45-0.60]; P < .001).
Conclusions and relevance: Among patients with AF, the strategy of catheter ablation, compared with medical therapy, did not significantly reduce the primary composite end point of death, disabling stroke, serious bleeding, or cardiac arrest. However, the estimated treatment effect of catheter ablation was affected by lower-than-expected event rates and treatment crossovers, which should be considered in interpreting the results of the trial.
CAST I & II
Cardiac Arrhythmia Survival Trial (CAST I and II) data. Association between ease of suppression of ventricular arrhythmia and survival. Goldstein et al. Circulation. 1995
Background: We tested the hypothesis that patients whose ventricular arrhythmias are easy to suppress have a lower rate of arrhythmic death, defined as arrhythmic death and nonfatal cardiac arrest, the primary end point in the Cardiac Arrhythmia Suppression Trials (CAST-I and CAST-II), than patients whose ventricular arrhythmias are hard to suppress. In addition, we evaluated the association between ease of suppression of ventricular arrhythmias and mortality of all causes.
Methods and results: CAST-I investigated the effect on arrhythmic death of ventricular premature depolarization (VPD) suppression achieved by three drugs, encainide, flecainide, and moricizine, at two different dose levels; CAST-II investigated the same effect, using moricizine alone at three dose levels. If suppression was achieved, patients were randomized to the effective active drug or corresponding placebo. To examine the independence of easily suppressed ventricular arrhythmias as a predictor of arrhythmic death, we adjusted statistically for other variables that were related both to ease of suppression and arrhythmic death. Patients with ventricular arrhythmias (n = 1778) that were easy to suppress had fewer arrhythmic deaths during follow-up than those with ventricular arrhythmias that were hard to suppress (n = 1173) (relative risk, .59; P
CASTLE-AF
Catheter Ablation versus Standard Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) Trial.
Background: Mortality and morbidity are higher among patients with atrial fibrillation and heart failure than among those with heart failure alone. Catheter ablation for atrial fibrillation has been proposed as a means of improving outcomes among patients with heart failure who are otherwise receiving appropriate treatment.
Methods: We randomly assigned patients with symptomatic paroxysmal or persistent atrial fibrillation who did not have a response to antiarrhythmic drugs, had unacceptable side effects, or were unwilling to take these drugs to undergo either catheter ablation (179 patients) or medical therapy (rate or rhythm control) (184 patients) for atrial fibrillation in addition to guidelines-based therapy for heart failure. All the patients had New York Heart Association class II, III, or IV heart failure, a left ventricular ejection fraction of 35% or less, and an implanted defibrillator. The primary end point was a composite of death from any cause or hospitalization for worsening heart failure.
Results: After a median follow-up of 37.8 months, the primary composite end point occurred in significantly fewer patients in the ablation group than in the medical-therapy group (51 patients [28.5%] vs. 82 patients [44.6%]; hazard ratio, 0.62; 95% confidence interval [CI], 0.43 to 0.87; P=0.007). Significantly fewer patients in the ablation group died from any cause (24 [13.4%] vs. 46 [25.0%]; hazard ratio, 0.53; 95% CI, 0.32 to 0.86; P=0.01), were hospitalized for worsening heart failure (37 [20.7%] vs. 66 [35.9%]; hazard ratio, 0.56; 95% CI, 0.37 to 0.83; P=0.004), or died from cardiovascular causes (20 [11.2%] vs. 41 [22.3%]; hazard ratio, 0.49; 95% CI, 0.29 to 0.84; P=0.009).
Conclusions: Catheter ablation for atrial fibrillation in patients with heart failure was associated with a significantly lower rate of a composite end point of death from any cause or hospitalization for worsening heart failure than was medical therapy.
International retrospective study
Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group studyRoderick Tung et al. Heart Rhythm. 2015
Background: The impact of catheter ablation of ventricular tachycardia (VT) on all-cause mortality remains unknown.
Objective: The purpose of this study was to examine the association between VT recurrence after ablation and survival in patients with scar-related VT.
Methods: Analysis of 2061 patients with structural heart disease referred for catheter ablation of scar-related VT from 12 international centers was performed. Data on clinical and procedural variables, VT recurrence, and mortality were analyzed. Kaplan-Meier analysis was used to estimate freedom from recurrent VT, transplant, and death. Cox proportional hazards frailty models were used to analyze the effect of risk factors on VT recurrence and mortality.
Results: One-year freedom from VT recurrence was 70% (72% in ischemic and 68% in nonischemic cardiomyopathy). Fifty-seven patients (3%) underwent cardiac transplantation, and 216 (10%) died during follow-up. At 1 year, the estimated rate of transplant and/or mortality was 15% (same for ischemic and nonischemic cardiomyopathy). Transplant-free survival was significantly higher in patients without VT recurrence than in those with recurrence (90% vs 71%, P<.001). In multivariable analysis, recurrence of VT after ablation showed the highest risk for transplant and/or mortality [hazard ratio 6.9 (95% CI 5.3-9.0), P<.001]. In patients with ejection fraction <30% and across all New York Heart Association functional classes, improved transplant-free survival was seen in those without VT recurrence.
Conclusion: Catheter ablation of VT in patients with structural heart disease results in 70% freedom from VT recurrence, with an overall transplant and/or mortality rate of 15% at 1 year. Freedom from VT recurrence is associated with improved transplant-free survival, independent of heart failure severity.
Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;
Background: In patients with ventricular tachycardia (VT) and a history of myocardial infarction, intervention with an implantable cardioverter defibrillator (ICD) can prevent sudden cardiac death and thereby reduce total mortality. However, ICD shocks are painful and do not provide complete protection against sudden cardiac death. We assessed the potential benefit of catheter ablation before implantation of a cardioverter defibrillator.
Methods: The Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH) study was a prospective, open, randomised controlled trial, undertaken in 16 centres in four European countries. Patients aged 18-80 years were eligible for enrolment if they had stable VT, previous myocardial infarction, and reduced left-ventricular ejection fraction (LVEF; 30%). Patients were followed up for at least 1 year. The primary endpoint was the time to first recurrence of VT or ventricular fibrillation (VF). Analysis was by intention to treat (ITT).
Findings:107 patients were included in the ITT population (ablation group, n=52; control group, n=55). Two patients (one in each group) withdrew consent immediately after randomisation without any follow-up data and one patient (ablation group) was excluded because of a protocol violaton. Mean follow-up was 22·5 months (SD 9·0). Time to recurrence of VT or VF was longer in the ablation group (median 18·6 months [lower quartile 2·4, upper quartile not determinable]) than in the control group (5·9 months [IQR 0·8–26·7]). At 2 years, estimates for survival free from VT or VF were 47% in the ablation group and 29% in the control group (hazard ratio 0·61; 95% CI 0·37–0·99; p=0·045). Complications related to the ablation procedure occurred in two patients; no deaths occurred within 30 days after ablation. 15 device-related complications requiring surgical intervention occurred in 13 patients (ablation group, four; control group, nine). Nine patients died during the study (ablation group, five; control group, four).
Interpretation: Prophylactic VT ablation before defibrillator implantation seemed to prolong time to recurrence of VT in patients with stable VT, previous myocardial infarction, and reduced LVEF. Prophylactic catheter ablation should therefore be considered before implantation of a cardioverter defibrillator in such patients.