Weight and body water, Hypernatraemiaemia, Osmolarity, Hyponatraemia, getting Sodium, and acid-base-balance. Hypo abd hyperkalemia.
Section A: Weight and fluid, Section B: Electrolyte, Section C: Acid-base balance.
Contents
Section A: Weight Fluid balance and hypernatraemia
A1. Body weight and fluid
A2. Hypernatraemia
A3. Getting water
A4. Osmolar gap
Section B: Hyponatremia:
B1. Getting sodium, Sodium preparations, Potassium preparations and Other means
B2. Clinical categories, Clinical approach, Laboratory tests, Sodium replacement
B3. Sodium replacement
Section C: Acid-base balance
C1. Acid-base balance
C2. Compensation
C3 Anion gap
C4. Delta ratio
C5. Urinary anion gap
C6. Lactic acidosis
C7. Management of metabolic acidosis
C8. Metabolic alkalosis
C9. Hypokalaemia and
C10. Hyperkalemia
_____________________________________
Section A: Fluid balance & hypernatraemia
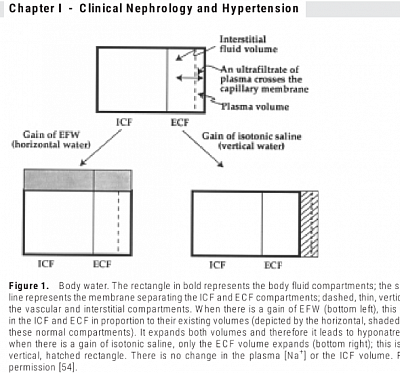
A1. Body weight and Body water 💦
- TBW (Total Body Water) is one-third ECF (Extracellular fluid) & two-thirds ICF (intracellular fluid). TBW is 0.6XBWt (body weight in Kg) (male), 0.55XBWt Kg (female).
- ECF is one third blood volume (BV) & two third interstitial fluid (ISF).
- Plasma volume is (1- haematocrit)xBV, Or (1-Hct)x1/3 ECV, Or (1-Hct)x(1/9) TBW.
- Water Deficit L= (Na-140L)xTBW/140
- TBW = Wt in Kg x 0.6 (male), Wt in Kg x 0.55 (female).
- Ideal BWt (kg) = (Ht"-60)x2.3+50 in male, (Ht"-60)x2.3+45.5 female,
- IBWt is better in obesity & drug dosing. (hight in inches 1"= 2.54 cm).
_____________________________________
A2. Hypernatraemia
Hypernatraemia indicates mainly water deficit. (Iatrogenic, in tube feeding patients or in ICU cases). You need to replace water as D5 or Half NS to reduce Na level.
- Monitor Na changes as follows: (Target Na- Serum Na) should be <10 mmol/L (preferably 5-6) in first 24 hours.
- 6 hourly UE check: rate of Na decrease ~Initially <1 mmol/L/hr may be allowed in first 6 hours.
- Subsequently <0.5 (net <10 mmol/L in 24 hrs).
_____________________________________
A3. Getting water.
- Dextrose 5%
When you infuse D5 the glucose is rapidly metabolised. The net effect is of administering pure water, so it is distributed throughout the total body water. Each compartment receives fluid in proportion to its contribution to the TBW (ie 2/3rd to ICF and 1/3rd to ECF). So, with 1L of D5, you supply 110 mls to blood volume. Rest goes to cells (670 ml) and ISF (220 ml).
Dextrose 5% is essentially treated by body as water, and a significant proportion (2/3) moves intracellularly.
- Normal Saline
Sodium is mainly extracellular. So, NS remains in ECF. The water distributes between the ISF & the BV in proportion to their volume. Plasma osmolality will remain unchanged because normal saline is isosmotic.
Normal saline is a ‘replacement fluid’ (meaning ECF replacement) because it adds only to the ECF volume. Only about a third remains intravascularly.
- Hypertonic saline 3%
Hypertonic saline 3% has an osmolality (about 900 mOsm/l) three times that of plasma.
- 8.4% NaHCO3 solution
This is a molar solution of NaHCO3. Dissociation into two particles in solution results in a solution with an osmolality of 2,000 mOsm/kg. This is about 7 times the plasma osmolality.
- Plasma Protein Solution
Plasma protein solution is a colloid and is distributed only to the intravascular fluid. The tonicity is unaltered.
Plasma protein solutions (eg 5% human albumin, Gelafundin 4% gelatin) are excellent for replacing intravascular volume. ISF and ICF will not be replenished.
- Water- You cannot infuse water, it will cause plasmolysis.
Overview
1. Dextrose 5% is essentially treated by body as water, and a significant proportion (2/3) moves intracellularly.
2. Normal saline is a ‘replacement fluid’ (meaning ECF replacement) because it adds only to the ECF volume. Only about a third remains intravascularly.
3. Plasma protein solutions (eg 5% human albumin, Gelafundin 4% gelatin) are excellent for replacing intravascular volume. ISF and ICF will not be replenished.
_____________________________________
A4. Osmolar Gap
1. Osmolality is measured in the laboratory by machine called osmometer. The unit of osmolality is mOsm/L.
2. Osmolarity is calculated. as follows:= (2x [Na+K] ) + glucose mmol/l + BUN mmol/l.
3. Osmolar gap = Osmolality - Osmolarity (Normal <10)
4. In certain conditions, significant amounts of abnormal substances are present in plasma which contribute to the increased osmolality. However, the calculated osmorarity remains unaltered.
Consequently the osmolar gap is increased.
Examples are alcohol Poisoning and ethylene glycol Poisoning. It helps in diagnosis of these conditions.
_____________________________________
Section B: Hyponatraemia & getting Sodium
B1. Clinical considerations
- Mild: 130-134, moderate: 125-129, profound: <125
- Hypovolaemic
- Euvolemic
- Hypervolaemic
Also can be hypotonic, isotonic & hypertonic.
- Acute: <48 hours
- Chronic: >48 hours
- Corrected Na = SNa + 2.4x{(S Glucose - 5.5mmol)/5.5mmol/l} (5.5 mmol = 100 mg)
B2. Essential tests
- Urine Na: N 20 - 30,
- Serum Osmolality: N 275 - 300
- Urine Osmolality: N 100 - 800
B3. Hyponatraemia: Clinical categories
You need to find out the cause in order to correct it.
Diagnose clinically -You need to classify into 4 categories:
Dilutional, Depletional, Nutritional, SIADH/Pseudo hyponatraemia & CSWS.
You can diagnose by History & physical exam, then confirm by simple test.
Clinical approach:
Fluid overload from any causes including hypothyroidism, indicates Dilutional: you can use frusemide for increasing sodium level. If your patient is ADL dependant and emaciated, possibly Nutritional deficiency: you need to replace. If your patient is dehydrated, it indicates Depletional: Needs replacement of sodium as written below.
If your patient is euvolaemic, elderly or stroked out or taking multiple drugs, you may suspect SIADH or CSWS; Then you may resort to water restriction to raise sodium level.
Laboratory tests
For any hyponatraemia, you need to do:
Serum osmolality urine osmolality urine sodium
Interpretation
This is for deduction of your categories as follows:
Low serum osmolality, with or without low urine sodium & osmolality >> dilutional.
Normal or high serum osmolality, high urine sodium, normal urine osmolality >> depletional from urine loss (Diuretic use, Bartter or Gitelmen syndrome, Addison's disease etc). High or normal serum osmolality, low urine sodium >> depletional through gut loss or sweetng in desert or at Marathon. High serum osmolality, low urine sodium, high urine osmolality >> nutritional deficit. Low serum osmolality, normal urine sodium, with or without high urine osmolality >> SIADH, Low serum osmolality, high urine sodium, high urine osmolality >>CSWS.
B4. Management:
a) Getting sodium
Sodium preparations, Potassium preparations and Other means
b) Sodium replacement
a) Getting Sodium
You can get Sodium through -
Sodium preparations, potassium preparations and other means.
From Sodium preparations:
- 1gm NaCl= 17 mmol Na (43x2/5)
- 1 gm NaHCO3= 11 mmol Na (43x1/4)
- 1 sachet ORS mixed with 250 ml of water contains 75 mmol/L Na.
- 1 liter iv 0.9% saline solutions= 154 mmol Na
- 1 liter iv 3% saline= 514 mmol Na
- 1 ml 8.4% NaHCO3= 1 mmol Na & 1 mmol of HCO3.
------------
How do you get these?
- 1gm Sodium, Na = 43mmol [1000/23]
- Sodium Chloride tablet contains 2/5th Na [23:35]
- Sodium Bicarb tablet contains 1/4th Na [23/(23+1+12+48)
-------------------
- From KCl preparation:
- 1 mmol K= 1 mmol Na
Administration of K causes Na to increase in ECF through exchange from IC space in equal amount.
- 600 mg of Span-K contain 8 mmol of K
- 10 ml 10% Mist KCl = 1 gm KCl =13 mmol K
- 10 ml 7.4% iv KCl = 10 mmol K
By other means:
- Water restriction in SIADH.
- Free water excretion with frusemide,
- ADH antagonism with V2R blocker
b) Sodium replacement
Having gotten your diagnosis, if you need to get sodium, follow the sequences below:
-
- Determine the Na requirements for next 24 hours in mmol to raise serum Na level to your target level by the formula: (Target Na - SNa)x0.6xBWkg [women 0.55].
- (Target Na- SNa) shoud be<10 mmol/L, (preferably 5-6) in first 24 hours.
- Determine rout: if IV, calculate the fluid and sodium amount as above, to give over 24 hours.
- If oral, calculate as above, as daily dose.
- Six-hourly UE check: rate of Na increase: Initially<1 mmol/L/hr may be allowed in first 6 hours,
- Subsequently <0.5 (net <10 mmol/L in 24 hrs.
___________________________________
Section C: Acid base balance
www.anaesthesiamcq.com
Contentsp
C1. Acid-base balance
C2. Compensation
C3. Anion gap
C4. Delta ratio
C5. Urinary anion gap
C6. Lactic acidosis
C7. Management of metabolic acidosis
C8. Metabolic alkalosis
C9. Hypokalaemia and hyperkalemia
_____________________________________
C1. Acid-base balance
- When serum bicarbonate level is less than 22mmol/L (KDIGO, 2009) or higher than 28 mmol/L, know the clinical details of the patient. Then do ABG- Arterial blood gas analysis: pH indicates whether you are dealing with acidosis or alkaloss:
- pH <7.35, Acidosis:
- Look for HCO3<24: indicates METABOLIC acidosis.
- Look for pCO2>40: indicates Respiratory acidosis.
- pH >7.45, Alkalosis:
- Look for pCO2<40: indicates Respiratory alkalosis.
- Look for HCO3>28 :indicates Metabolic alkalosis.
_____________________________________
C2. Compensations- metabolic and respiratory
In Metabolic Acidosis-
- look for Respiratory compensation and mixed pattern:
- Compensation - Expected pCO2 = 1.5 x [HCO3] + 8 (actual pCO2 within the range of +/- 2).
- If beyond the range, indicates mixed Respiratory pattern; i.e. actual pCO2> Ex pCO2, indicates mixed respiratory acidosis;
- Actual pCO2 < Ex pCO2, indicates mixed respiratory alkalosis.
In Metabolic alkalosis-
- look for Respiratory compensation and mixed pattern:
- Compensation is Expected pCO2 = 0.7 [HCO3] + 20 (range: +/- 5).
- If beyond the range - indicates mixed respiratory acidosis or alkalosis as above.
In Acute Respiratory Acidosis:
- look for Metabolic compensation.
- Expected [HCO3] = 24 + { (Actual pCO2 - 40) / 10 }.
- If beyond the range - indicates mixed metabolic acidosis or alkalosis.
In Chronic Respiratory Acidosis -
- look for Metabolic compensation.
- Expected [HCO3] = 24+4{(Actual pCO2 - 40) / 10}.
- If beyond the range - indicates mixed metabolic acidosis or alkalosis as above.
In Acute Respiratory Alkalosis -
- look for Metabolic compensation.
- Expected [HCO3] =24-{(40-Actual pCO2)/10}.
- If beyond the range - indicates mixed Respiratory Acidosis /alkalosis.
In Chronic Respiratory Alkalosis-
- look for Metabolic compensation.
- Expected [HCO3] = 24-5{(40 - Actual pCO2)/10)} (range: +/- 2).
- If beyond the range - indicates mixed Respiratory Acidosis or alkalosis.
_____________________________________
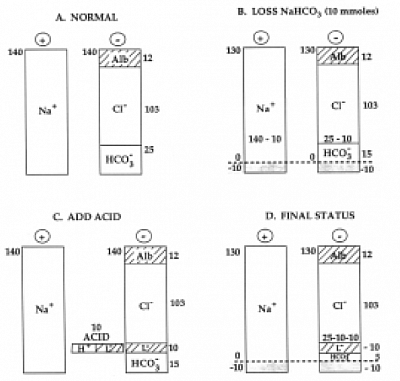
C3. Anion Gap: For Metabolic Acidosis
- you need to use the Anion gap (AG).
- AG=(Na-[Cl+HCO3]).
- Reference range is 8 to 16 mmol/l (Avg 12).
- Albumin correction: Hypoalbuminaemia causes a low anion gap.
- Albumin corrected Anion gap={AG+(40-ALB)/4}.
HAGMA
- High Anion Gap Metabolic Acidosis (AG>12)
- When you acquire exo or endogenous acids. (renal failure, liver failure, heart failure, respiratory failure, sepsis, starvation, ketosis. Lactic acidosis; drugs, Toxin, ethanol, glycol, etc).l
NAGMA
- Normal Anion Gap Metabolic Acidosis (AG<13):
- When you lose alkali through urine or gut. (Renal Tubular acidosis, Addison's disease, diarrhoea, Polyposis coli, etc).
_____________________________________
C4. The Delta Ratio-
In metabolic acidosis you need to use Delta ratio (Delta Gap / Delta bicarbonate) =(AG corrected – 12)/(24 - HCO3).
Delta Ratio helps you to find NAGMA-HAGMA relationship as follows:
- < 0.4 - Hyperchloraemic normal anion gap acidosis
- 0.4 to 0.8 - Combined high AG and normal AG acidosis
- 1 - Common in DKA due to urinary ketone loss
- 1 to 2 - Typical pattern in high anion gap metabolic acidosis
- > 2 Check for either a co-existing Metabolic alkalosis.
_____________________________________
C5. Urine anion gap -
in NAGMA, use urinary anion gap to see if low bicarbonate is due to urinary loss or GIT loss.
- The cations in urine are Na+, K+, NH4+, Ca++ and Mg++.
- The anions in urine are Cl-, HCO3-, sulphate, phosphate and some organic anions.
- Only Na+, K+ and Cl- are commonly measured in urine.
- The others are the unmeasured anions (UA) and cations (UC).
- Because of the requirement for electroneutrality, total anion charge always equals total cation charge.
- Therefore: (Na+ K+ UC) - (CL+ UA) =0, or (Na+ K+ UC) = (CL+ UA) or (Na+ K) = CL or (Na+K) - Cl=0.
- Normal Urinary Anion Gap, [Na+]+ [K+] - [Cl-] = 0.
- When you get a positive value, it indicates renal loss of HCO3, i.e. RTA.
- When you have a negative value, it's gut loss of HCO3, i.e. GE. (negut for gut).
_____________________________________
C6. Lactic acidosis
In metabolic HAGMA, when routine test shows Lactate above reference range, it's known as lactic acidosis.
- It happens when glycolysis takes place in the Hexose monophosphate shunt, so called Anaerobic glycolysis. This obviously can happen in hypoxic condition causing hypoxic or type-A.
- It also happen in tissue toxic state when mitochondrial respiration is depressed where Krebcycle take place. Thus, as pyruvate being unable to enter the krebs cycle, is converted to Lactate producing lactic acidosis. This is known as Non hypoxic or type B.
- Hypoxic condition happens in respiratory failure, heart failure, circulatory failure (shock), drowning, severe anaemia, methaemoglobinaemia, CO poisoning etc.
- Non-hypoxic tissue toxicity happens in poisoning, severe sepsis, liver failure, renal failure, starvation, diabetic keto acidosis, malignancies, drugs like merformin, anesthetic agents etc.
- Warburg phenomena: In malignancies, some time malignant cells shift glycolysis to HMP pathway from aerobic glycolysis, thus producing type B lactic acidosis.
- d-lactic acidosis: physiological lactic acid is a levo isomers. In certain situations of intestinal tract, i.e. diverticulitis, intestinal bypass surgery, megacolon, intestinal bacteria produce dextro isomers of Lactate, d-lactic acid.
D-Lactate is not broken down by enzyme Lactate dehydrogenase (LDH), as such, it accumulates leading to lactic acidosis (a HAGMA), that is not detected by usual test for Lactate. On suspicion special test needs to be done.
The distinctive features are that of encephalopathy, that lead to suspicion.
_____________________________________
Overview of metabolic acidosis -
- Initial investigations are serum urea electrolytes bicarbonate, Creatinine and glucose.
- When HCO3 is less than 22 mmol/L, do ABG, LFT for Albumin. Subsequently do Serum Lactate and ketone.
- Step 1: Metabolic acidosis can be divided into two groups based on the anion gap (AG): HAGMA, high anion gap acidosis and NAGMA, normal anion gap (hyperchloraemic) acidosis.
- STEP 2:
- for HAGMA, do Delta Ratio and analyze as above;
- for NAGMA, do Urine anion-gap, to find out if loss of base is through the kidney (eg renal tubular acidosis) or GI tract.
- A positive UAG suggests renal loss of bicarbonate (ie renal tubular acidosis).
- A negative UAG suggests GIT loss of bicarbonate (eg diarrhoea).
_____________________________________
C7. Management -
- Treatment of underlying causes,
- Replacement of measured bicarbonate,
- In severe cases (HCO3 <10) Haemodiafiltration (CVVHDF), along with HCO3 replacement.
Replacement of HCO3:
- HCO3 15-22 mmol/l: use oral tablet,
- HCO3 10-14, use IV HCO3 after calculating the deficit. We use KT Woo (ISN-CM) formulae to calculate [HCO3 deficit= [(24-sHCO3)X BWKg/3].
- Infuse HCO3 half amount over 2-4 hours, then check again and repeat the sequence if needed.
- 8.4% NaHCO3 Solution ( 1ml = 1 mmol of HCO3 and 1 mmol of Na): you can infuse 50 ml in 100 ml of water for injection over 1 hour in Acute situation. Look out for Hypocalcaemia and tetany (because of precipitation of Ca).
- Isotonic Bicarbonate solution: 75 ml of 8.4% NaHCO3 + 425 ml of D5, makes 1 pint of Isotonic Bicarb. This is because 75 mmol Na and 75 mmol HCO3 is 150 mmol in 1 pint (500 ml). In 1 liter 300 mmol (2×75×2), So Isotonic. Dextrose is rapidly f ly used up system. This can be given Q6, Q8 or Q12, according to your decision.
- You can also use more aggressive Mark Manuals formulae [HCO3 deficit= (24-sHCO3)X BWKg X 0.4], replacing the amount in 3-6 hours. You need to check electrolytes, HCO3 & calcium frequently for Hypocalcaemia and Hypocalcaemia.
- HCO3 <10, consider CRRT.
_____________________________________
C8. Metabolic alkalosis -
- You need to pay attention to the clinical assessment to make obvious diagnosis, i.e. contraction alkalosis, drug (diuretic) induced, endocrine (Cushing/Conn's), renal causes (Gitelman syndrome, Bartter syndrome, Liddle's syndrome etc).
- Next to do: Urine Chloride:
a) <20 mmol/l, indicates Chloride sensitive metabolic alkalosis responds to saline infusion, caused by vomiting or diuretics.
b) >20 mmol/L, indicates Chloride resistant metabolic alkalosis due to endocrine causes or renal.
_____________________________________
_____________________________________
C9. Hypo and hyperkalemia - Link to the 🆓 resource and eBooks.
Hypokalemia and hyperkalemia management
Electrolyte imbalance, in particular hypo and hyperkalemia are very common in hospital practice, mandating urgent attention.
© Hypokalemia
In general, they can be corrected in non ICU /HDU settings in general wards. K of 2.5 without any simptom like AF/af, can be managed in general ward by slow IV replacement with the help of good nurses in renal and non-renal sides. Beyond that you need cardiac monitored iv replacement in HDU setup.
KCl tablet 600 mg contain 8 mmol K, Mist KCl 7 mmol K in 10 ml. Use your judgement for dosages.
IV KCl 10 ml contain 10 mmol K. Dilute in 100 ml D5 and give over 2 hours. Never give faster in less than 1 hour, otherwise it will lead to VF & patient's collapse.
© Hyperkalemia
The case of hyperkalemia is more challenging in hospital setup, in particular, that leads to ventricular arrhythmias which could be life terminating. In this situation a high level of k mandates HDU /ICU admission.
(A toddler in general ward mwith gross anasarca who was given Fru-lac outside. Spironolactone is contraindicated in AKI - in this case PSGN.
The toddler was edematous and restless. Post admission K was 6. The toddler was transferred to pediatric ICU and received sulbutamol inhaler. This is an appropriate case of ICU management).
Mild asymptomatic hyperkalemia
Use oral Resonium or Lokelma or Patiromar (chronic situation) at your judgemental dosages.
Symptomatic or K>5.8 mmol/l,
Use Hyperkalemia kit according to your hospital protocol with Insulin dextrose (1 unit/5-10 gm), and iv Calcium gluconate 10 ml, repeat as required. Salbutamol nebuliser in sutuation base states.
Denying treatment for Hypokalaemia without simptom in general ward, and transferring to HDU /ICU, is actually not acceptable.
—--------————----------------------