Renal support for haemodynamically unstable patients
Critical Care Nephrology
Contents
- Indications
- Modalities
- Differences among CRRT modalities
- Principles of CRRT
- Consumables of CRRT
- Replacement Fluid of CRRT
- CRRT prescriptions
- Other blood purification methods
_____________________________________
1. Indications
Traditional intermittent hemodialysis often causes hemodynamic instability in the critically ill. Continuous renal replacement therapy (CRRT) was developed in the 1980s in an effort to provide artificial kidney support to patients who could not tolerate traditional hemodialysis. The earliest forms of CRRT used arterial and venous access and depended on the patient’s mean arterial pressure to push blood through the filter. This technique was rarely successful for patients in shock. The current techniques of veno-venous CRRT involves blood pump. Most CRRT delivered today uses veno-venous access and an external blood pump to maintain adequate flow through the filter. As CRRT techniques have become more effective, its use has increased dramatically. In Australia 90% of patients in an ICU with acute renal failure receive CRRT, in Europe about 50%.
Indications for hemodynamically unstable patients and in ICU setup:
- Fluid overload
- Acute renal failure
- Chronic renal failure
- Life-threatening electrolyte imbalance
- Major burns with compromised renal function
- Drug overdose
_____________________________________
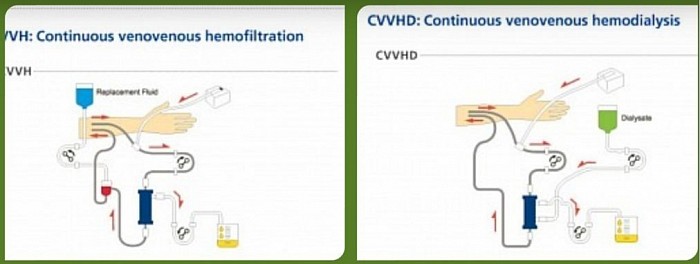
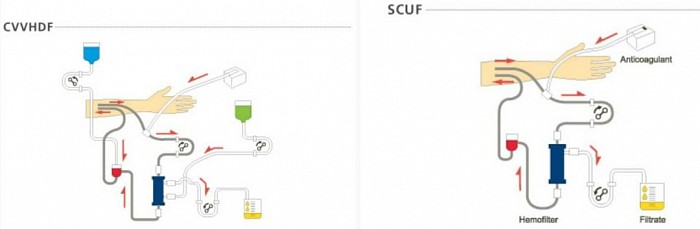
2. Modalities
The renal support is provided by so called Extracorporeal blood purification through Continuous Renal Replacement Therapy (CRRT). The modalities are as follows:
- CVVH - CVV hemofiltration
- CVVHDF - CVV hemodiafiltration
- CVVHD - CVV hemodialysis
- SCUF - Slow continuous Ultrafiltration
- MARS - Molecular Adsorption Recirculating System (Liver dialysis)
- Haemoperfusion
- Plasmapheresis
- Double Filtration Plasmapheresis and
- Lipopheresis
Brief description of CRRT modalities
- Slow Continuous Ultrafiltration (SCUF) - fluid removal only over 6 - 12 hours.
- Continuous Veno-venous Hemofiltration (CVVH) - Convection only. +/- UF.
- Continuous Veno-venous Hemodialysis (CVVHD)- Diffusion only.+/- UF.
- Continuous Veno-venous Hemodiafiltration (CVVHDF) - Convection and Diffusion. +/- UF
_____________________________________
Diagrams of CRRT modalities
3. Differences between CRRT, HD and CVVH, CVVHD (link)
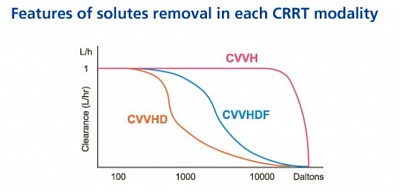
The solute removal varies in each modalities to some extent as shown in the figure. Even though, dialysis offers diffusion of smaller particles, larger molecular weight particles are better removed by CVVH and CVVHDF, more than CVVHD. Two reasons: use of larger pore hemofilter allowing removal of 40kD+ particles and Solvent drag by draining water during ultrafiltration through highflux membrane. (when you talk about particle removal, you say high efficiency hemofilter and when you say about water removal you say highflux membrane). When you remove 110kD partocle like Lamda chain, you say high cutoff membrane for high cutoff dialysis for MM.
Similarly, for plasmapheresis, where you remove plasma protein, you use Plasma filter.
4. Principles of CRRT
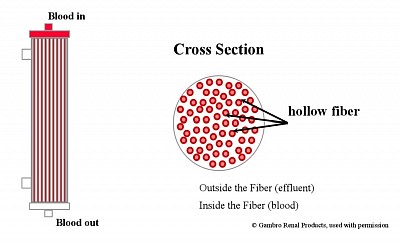
Semipermeable Membranes- Highflux membrane filter, High-efficiency membrane Filter. Diffusion through membrane along concentration gradient - using High efficiency membrane.Ultrafiltration through large pores with fluid replacement - Highflux filter.Convection by solute drag through large UF andReplacement using infusible replacement fluid - (bad fluid is replaced by good fluid.Adsorption on the surface of membrane of particles.Vascular Access and the Extracorporeal Circuit - dual lumen temporary dialysis catheters in IJ or Femoral vein.
The solute removal varies in each modalities to some extent as shown in the figure. Even though, dialysis offers diffusion of smaller particles, larger molecular weight particles are better removed by CVVH more than CVVHDF.
_____________________________________
5. Consumables of CRRT
a) Fluids Used in CRRT
- Replacement Fluids (CVVH, CVVHDF)
- Dialysate (CVVHD, CVVHDF)
- None in SCUF, only fluid is removed over 4 to 6 hours time.
- Aquapheresis: only fluid is removed with slow blood flow and slow UF rate of 50 to 100 ml per hour.
b) Anticoagulation & CRRT
- Heparin
- Prostacyclin
- Citrate
- No Anticoagulation
c) Replacement fluid (custom made)
- Replacement flow rates 20 – 40 mL/kg/hr. Higher the volume, higher is the CRRT dose. But mortality benefit uncertain.
- Commercially prepared pre-mixed replacement fluid (hemosol, Prismasol, prismocitrate, PD fluid. In PD fluid use, monitoring blood glucose and appropriate insulin coverage is required).
- Custom replacement fluid compounded by the pharmacy:
Example:
- NS 700ml+
- 5% D 300 ml+
- 8.4% NaHCO3 30 ml+
- 10 ml 10% Ca gluconate+
- 0.5 ml 50% MgSO4 +/-
- 7.4% KCl 3-5 ml).
Final concentration: (Na 135 mmol/l, HCO3 30 mmol/l, Ca 2.5 mmol/L, K 3-5 mmol/l).
- Additives: KCl & KHPO as per protocol..
d) Dialysate:
- Same replacement fluid for CRRT is used as Dialysate fluid. (These are infusible).
Additives in Commercial replacement and Dialysate fluid-
- D5% & 3% NaCl
- The commercial fluid Na conc is 140 mmol/l.
- In case of hyponatremia, you need to add D5% (water) in required amount to reduce Na conc to nearest +5 mmol/l in the fluid.
- In case of hypernatraemia, you need to add required amount of 3%NaCl to increase Na conc to nearest -5 mmol/l in the fluid.
- 7.5%KCl and KHPO, 50% MgSO as per protocol.
- 10% Ca Gluconate, in Citrate Anticoagulation with Prismocitrate prereplacemment and PrismoCal postreplacement, as per protocol.
Dialysate:
Same replacement fluid for CRRT is used as Dialysate fluid. (These become infusible after passing through 2nd series of filters present in Haemodiafiltration machines, inbuilt).
____________________________________
6. Infusible Replacement Fluid composition -
Ionic composition: (in mmol/L & meq/L)H
Hemosol
- Calcium Ca: 1.75 /3.50
- Magnesium Mg: + 0.5 /1.0
- Sodium Na: 140 /140
- Chloride Cl: 109.5 /109.5
- Lactate: 3 /3Bicarbonate:
- HCO3- 32 /32
- Theoretical Osmolarity: 287 mOsm/l.
Prismosol with lactate
MultiBic with HCO3 35 (Maximum)
Phoxilium with Potassium (2), Phophate, and low bicarb (30)
MultiBic plus potassium, Phosphate and bicarb 35.
Replacement fluid
Replacement flow rates 20 – 40 mL/kg/hr.
Higher the volume, higher is the CRRT dose. But mortality benefit uncertain.
Commercially prepared pre-mixed replacement fluid (hemosol, Prismasol, prismocitrate, PD fluid. In PD fluid use, monitoring blood glucose and appropriate insulin coverage is required).
Custom replacement fluid compounded by the pharmacy (example: NS 700ml+ 5%D 300 ml, 8.4% NaHCO3 30 ml + 10 ml 10% Ca gluconate + 0.5 ml 50% MgSO4 +/- 7.4% KCl 3-5 ml). (Na 135 mmol/l, HCO3 30 mmol/l, Ca 2.5 mmol/L, K 3-5 mmol/l).Additives: KCl & KHPO.
____________________________________
7. CRRT prescription
____________________________________
- Bood flow rate (Qb) - 150-200,ml/ min
- Replacement flow rate - for CVVH & CVVHDF, 20-40 ml/kg/hour (this is considered as dose of CRRT as noted above).
- Ultrafiltration rate - Machines remove Ultrafiltration (replacement amount + target hourly net UF) as hourly effluent, by it's peculiar volumetric control as described by the vendors.
- In case of CVVHDF, the infused Dialysate (for example 1000 ml/hour) also comes out as effluent.
- The volumetric machine, use the replacement fluid as Dialysate and thus it adds additional Dialysate amount (+1000 ml/hr) in the Dialysate port of hemofilter.
- Thus total infusate is increased by that amount of Dialysate.
- In essence, machine draws as infusate, the amount of hourly replacement as decided by you + dialysis amount as decided by you.
- The total infusate is then fractionated by the machine as pre &/or postfilter replacement and dialysate, while the volumetric machine adjust the transmembrane pressure in the haemofilter in order to ultrafilter amount equal to the replacement and the target UF.
- Thus maintaining the negative balance equal to the target UF.
- The effluent thus equals to the total UF and Dialysate that is discarded in the drain to maintain nature's water-cycle.
- Dialysate flow rate for CVVHD & CVVHDF -
- As described above, fractionated by the machine from the replacement solution to run through the haemofilter as dialysate.
- Normally this does not exceed 50% of the replacement flow.
_____________________________________
8. Other extracorporeal blood purification process
- Plasmapheresis (PP)
- DFPP (Double filtration PP)
- MARS (Molecular Adsorption Recirculating System). Analytical study from SGH.
- Haemoperfusion
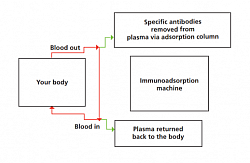
- Immunoadsorption
- Lipopheresis
- Other hybrid methods
________________________________
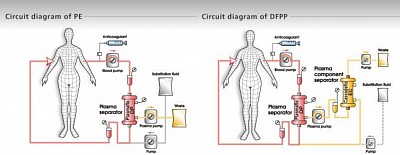
PLASMAPHERESIS, DFPP
Plasmapheresis
Plasmafilter is used where plasma (along with the plasma proteins) is filtered and discarded, the volume discarded is replaced by human albumin 5% &/or FFP (fresh frozen plasma).
Blood flow is 200 ml/min,
No Dialysate
Use Plasma filter.
UF: Nil
1 to 1.5x plasma volume [(BW X 0.6 (female 0,55) X 0.33 x (1 - HCT) = 1 PLASMA VOLUME], is removed with equal amount of replacement fluid (as above) returned to the body.
Heparin: very tight fraction. I
Vascular Access: dual lumen dialysis catheter.
(Haematologist use centrifugation cycles to remove plasma, the packed Cells are diluted with replacement fluid, and returned via the same single lumen catheter).
________________________________
DFPP
Double filtration or cascade plasmapharesis.
Here, affluent from plasmafilter is flown through a Hemofilter in the 'A' blood port. Thus the plasma is filtered, and the filtrate (devoid of plasma proteins) is returned to the body through the 'V' line. The residual plasma proteins that exit through the Hemofilter, are discarded.
Thus, it reduces need for higher volume of FFP & Albumin.
This is now practiced in ABO incompetible transplant to remove blood group related antibody, in RPGN and vasculitis to remove the ANCA and NON-ANCA antibodies.
__________________________________
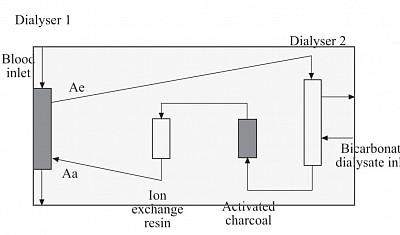
MARS (MOLECULAR ADSORBENT RECURCULATING SYSTEM)
Also known as liver dialysis, used as a bridging life support for acute fulminant liver failure often coupled with CRRT for concomitant AKI before liver transplant.
Components:
The system contains three
circuits:
the blood circuit,
an albumin circuit, and
an open–loop, single-pass
dialysis circuit.
Blood is passed through a
non–albumin–permeable
high flux dialysis membrane
(MARS–flux).
The albumin circuit, containing 20% albumin,
is passed through the MARS–flux and
subsequently regenerated by dialysis
against a bicarbonate–buffered
dialysate,
Followed by passage through two sequential
columns;
the first contains uncoated charcoal and
the second contains an anion exchanger
resin.
Prniciple:
- Albumin dialysis is used for adsorbing liver Toxin that are mostly protein bound. The Toxin in plasma are adsobed by albumin circulating in the high efficiency dialyser counter current to blood flow similar to Hemodialysis.
- The Toxin-bound albumin is then circulated in the second dialyser similar to blood flow and dialysed against bicarbonate dialysate to be gotten rid of the non-protein-bound Toxin, and
- then flowed through activated charcoal (AC) chamber for further adsorption of protein bound Toxins from albumin;
- Finally from the AC CHAMBER, the Toxin free albumin is flown to ion exchange resin chamber.
- From here the almost cleaned albumin is recirculated to the first initial dialyser to complete the cycle. The cycle continues for 6 to 8 hours.
Prescription :
- Qb blood flow 150-200 ml/min,
- Qa albumin flow 150 to 200 ml/ min,
- Dialysate flow 300 ml/min,
- Heparin very tight fraction at 250 unit per hour,
- UF nil.
- Duration 6 - 8 hours.
- Albumin dialysate: 600 ml of Salt poor human albumin (20%) is required. Frequency ranges from daily to every other days till OLTX is done or liver recovery occurs.
______________________________
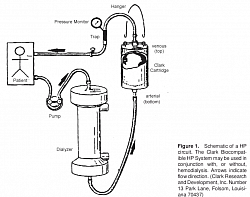
Haemoperfusion
You saw above the activated charcoal chamber, through which Toxin-bound albumin is flown to get the protein bound toxins adsobed by the AC.
In hemoperfusion, blood is flown AT a similar rate through chambers containing cells filled with ADSORBENT to which toxins, drugs and poisons are adsorbed, thus being removed from blood. Classic adsorbing substance is Activated charcoal.
Latest adsorbent is Polystyrene resin.
Poisons removed are, drug overdose, chemicals like parapet, heavy metals.
__________________________________
Hemoperfusion, Lipopheresis, Immunoadsorption
Haemopurfusion (HP), to remove toxin and drugs, is a process whereby blood is passed through a cartridge packed with a sorbent (activated charcoal or carbon, ion exchange resins or non-ionic macroporous resins. These are coated with porous polymer membranes. Blood flow Qb 300 ml/min. With heparin large dose.
LDL apheresis works by leading venous blood through a column containing beads coated with antibodies to apolipoprotein B (the main protein of LDL particles), dextran sulfate cellulose beads, modified polyacrylate beads, or by precipitating LDL with heparin at low pH, double membrane filtration or immunoadsorption utilizing Lp(a)-specific antibodies. In all cases (apart from polyacrylate absorption), plasma is separated from cells