Important medicines in cardiology
MEDICINES USED COMMONLY IN CARDIOLOGY
Contents
- Anti-lipid medicines
- Anti-coagulant and anti thrombotic
- Fibrinolytic
- Anti-arrhythmic
- Beta Blocker
- Diuretic
- Anti-Hypertensive
- Cardiotonic
- Vasoactive
- Corticosteroid
_______________________________
1. Anti-lipid medicines
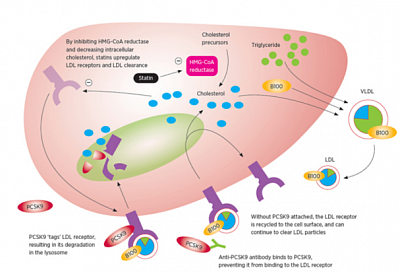
A. Monoclonal antibody Evolocumab (Repatha) used in:
1) Hereditary Familial Hyperlipidemia (HeFH), to reduce LDL-C. 140 mg SC q2weeks or 420 mg SC once monthly.
2) Cardiovascular Events: to reduce the risk of myocardial infarction, stroke, and coronary revascularization.
3) Primary Hyperlipidemia: Adjunct to diet, with primary hyperlipidemia, familial hyperlipidemia.
Homozygous Familial Hypercholesterolemia.
Indicated as an adjunct to diet and other LDL-lowering therapies (eg, statins, ezetimibe, LDL apheresis) for the treatment of patients with homozygous familial hypercholesterolemia (HEFH)
Measure LDL-C levels 4-8 wk after initiating,
No dose adjustment requiredSevere or end-stage renal disease (ESRD): reductions in PCSK9 levels in patients with severe renal impairment or ESRD receiving hemodialysis was similar.
Mechanism of action: Monoclonal antibody that binds to PCSK9 (proprotein convertase subtilisin/kexin type 9). LDL-C is cleared from the circulation preferentially through the LDL receptor (LDLR) pathway. PCSK9 is a serine protease that destroys LDLR in the liver, resulting in decreased LDL-C clearance and increased plasma LDL-C. PCSK9 inhibitors decrease LDLR degradation by PCSK9, and thereby improve LDL-C clearance and lower plasma LDL-C.
____________________________________
B. Atorvastatin (Atorvastatin Study) in Cardiovascular Disease Risk:
Statins, which inhibit cholesterol biosynthesis, have both pleiotropic and Low-Density Lipoprotein (LDL)-lowering properties. Atorvastatin is one of the choices for the primary and secondary prevention of cardiovascular disease and management of hypercholesterolemia.
____________________________________
2. Anti Coagulation
A. Warfarin (AHA)
Warfarin, a coumarin derivative, produces an anticoagulant effect by interfering with the cyclic interconversion of vitamin K and its 2,3 epoxide (vitamin K epoxide). Vitamin K is a cofactor for the carboxylation of glutamate residues to γ-carboxyglutamates (Gla) on the N-terminal regions of vitamin K–dependent PROTEINS. These proteins, which include the coagulation factors II, VII, IX, and X, require γ-carboxylation by vitamin K for biological activity.
CyP450: the gene coding for cytochrome P450, the hepatic enzyme responsible for oxidative metabolism of the warfarin S-ISOMER. Several genetic polymorphisms in this enzyme have been described that are associated with lower dose requirements and higher bleeding complication rates compared with the wild-type enzyme CYP2C9.
NSAID & ASPIRIN: Drugs such as ASPIRIN, nonsteroidal antiinflammatory DRUGS, penicillins (in high doses), and moxolactam increase the risk of warfarin-associated bleeding by inhibiting platelet function. Of these, aspirin is the most important because of its widespread use and prolonged effect. Aspirin and nonsteroidal antiinflammatory drugs also can produce gastric erosions that increase the risk of upper gastrointestinal bleeding.
Potentiation: erythromycin and some anabolic steroids potentiate the anticoagulant effect of warfarin are unknown. Sulfonamides and several broad-spectrum antibiotic compounds may augment the anticoagulant effect of warfarin in patients consuming diets deficient in vitamin K by eliminating bacterial flora and aggravating vitamin K deficiency.
Antithrombotic action: The suggestion that the antithrombotic effect of warfarin is reflected in lower levels of prothrombin forms the basis for overlapping heparin with warfarin until the PT (INR) is prolonged into the therapeutic range during treatment of patients with thrombosis. Because the half-life of prothrombin is ≈60 to 72 hours, ≥4 days’ overlap is necessary.
Anticoagulant action: anticoagulant action depends on reduction of 3 of the 4 vitamin K–dependent procoagulant clotting factors (II, VII, and X).
Metabolised by liver. Dose adjustment in renal failure not required, INR maintained by dose adjustment.
OTHER anticoagulant combination: Careful consideration needed when need to combine with DAPT. Enoxaparin can be used as bridging anticoagulant when warfarin is stopped for surgical intervention, as Enoxaparin can be stopped on the day of intervention.
____________________________________
B. Anti-Factor Xa (Apixaban) 2.5-5 upto10 mg bd. Renal impairment, no dose adjustment unless eGFR<15, OR Age>60, or chances of bleeding is high.
1- Stroke Prophylaxis with Atrial Fibrillation - nonvalvular.
2- Postoperative Prophylaxis of DVT/PE.
Indicated following hip or knee replacement surgery, for deep venous thrombosis (DVT) and pulmonary embolism (PE) prophylaxis.
3- Prevention of recurrent DVT, PE, 10 mg PO BID x 7 days, then 5 mg Bid. Recurrent DVT and PE following initial 6 months treatment for DVT and/or PE, 2.5 mg PO BID.
Renal impairment, including ESRD: No dose adjustment recommended.
Dosing adjustment with plasma anti-FXa activity level.
Switching between apixaban and anticoagulants other than warfarin: Discontinue one being taken, and begin the other at the next scheduled dose.
Switching from warfarin to apixaban: Discontinue warfarin and initiate apixaban when INR <2.0
Switching from apixaban to warfarin: Apixaban affects INR. So, discontinue apixaban and begin both a parenteral anticoagulant and warfarin at the time the next dose of apixaban. Discontinue parenteral anticoagulant when INR reaches an acceptable level.
Surgery/procedures: Discontinue at least 48 hr before elective surgery or invasive procedures with a moderate or high risk of unacceptable or clinically significant bleeding. Otherwise, discontinue at least 24 hr before.
____________________________________
C. Heparin
Inactivates factors IX, X (in low dose), XI and XII, and thrombin, and inhibits conversion of fibrinogen to fibrin. Also inhibits activation of factor VIII, in high dose.
Protein bound, Liver metabolism, nondialyable (as a unfractionated glucoseaminoglycan polymer).
1- PE:
Prophylaxis 5000 units SC q8-12hr,
Treatment 80 units/kg IV bolus, THEN continuous infusion of 18 units/kg/hr, OR 5000 units IV bolus, THEN continuous infusion of 1300 units/hr.
2- Acute Coronary Syndromes
PCI Without GPIIb/IIIa inhibitor: Initial IV bolus of 70-100 units/kg (target ACT 250-300 sec)
With GPIIb/IIIa inhibitor: Initial IV bolus of 50-70 units/kg (target ACT >200 sec).
3- STEMI
Patient on fibrinolytics: IV bolus of 60 units/kg (max: 4000 units), THEN 12 units/kg/hr (max 1000 units/hr) as continuous IV infusion. Dose should be adjusted to maintain aPTT of 50-70 sec.
4- Unstable Angina/NSTEMI: Initial IV bolus of 60-70 units/kg (max: 5000 units), THEN initial IV infusion of 12-15 units/kg/hr (max: 1000 units/hr).
Prolong one-stage prothrombin time.
Prevention of clot formation within venous and arterial catheters Use 100 units/mL.
_____________________________________
D. LOW MOLECULAR WEIGHT HEPARIN Tinzaparin)
Mechanism of work: LMWH; antithrombotic, that inhibits factor Xa by increasing inhibition rate of clotting proteases that are activated by antithrombin III.
Generally does not increase PT or PTT. Activity measured by anti Factor Xa assay.
Contraindicated with defibrotide mifepristone prothrombin complex concentrate.
Tinzaparin sodium Syringe 10,000 IU/ml Solution for injection in pre-filled syringe
1) Innohep® is a low molecular weight heparin indicated for treatment of deep-vein thrombosis (DVT) and pulmonary embolism (PE).
The product is available as a 2-mL multiple-dose vial containing 20,000 anti-factor Xa units of tinzaparin sodium per milliliter. The drug should be administered subcutaneously via a calibrated syringe once daily for at least six days until there is adequate anticoagulation with warfarin (an INR of at least 2 for two consecutive days). These recommendations are consistent with current treatment recommendations for acute DVT, which state that a minimum of five days of therapy with UFH or an LMWH should be provided while warfarin therapy is being adjusted to achieve an INR of 2-3.
2) Prophylaxis of venous thromboembolism in adult patients undergoing surgery, particularly orthopaedic, general or oncological surgery.
3) Prophylaxis of venous thromboembolism in non-surgical adult patients immobilised due to acute medical illness including: acute heart failure, acute respiratory failure, severe infections, active cancer, as well as exacerbation of rheumatic diseases.
4,500 anti-Xa IU given SC 12 hours before surgery and then once daily for as long as the patient is considered to be at risk of VTE.
4) Prevention of clotting in extracorporeal circuits during haemodialysis and haemofiltration in adults.
Duration of 4 hours or less:
A bolus injection of 2,000 to 2,500 anti-Xa IU at the start of dialysis.
Duration of more than 4 hours:
A bolus injection of 2,500 anti-Xa IU at the start of dialysis/filtration, followed by 750 anti-Xa IU/hour as a continuous infusion.
Interchangeability
No interchangeability with other LMWHs, because of pharmacologic differences.
Use in patients with a creatinine clearance level < 30 ml/minute is not recommended, as dosage in this population has not been established. Available evidence demonstrates no accumulation in patients with creatinine clearance levels down to 20 ml/min. When required in these patients, tinzaparin sodium administration can be initiated with anti-Xa monitoring, if the benefit outweighs the risk (see section 4.4: Renal impairment).
IRIS study: Innohep® in Renal Insufficiency Study (IRIS), suggesting that tinzaparin (Innohep®, LEO Pharma) may increase the risk for death, compared to unfractionated heparin (UFH) when used to treat elderly patients with renal insufficiency.
Contraindications
• Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
• Current or history of immune-mediated heparin-induced thrombocytopenia (type II) (see section 4.4).
• Active major haemorrhage or conditions predisposing to major haemorrhage. Major haemorrhage is defined as fulfilling any one of these three criteria: a) occurs in a critical area or organ (e.g. intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, intra-uterine or intramuscular with compartment syndrome), b) causes a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or c) leads to transfusion of 2 or more units of whole blood or red blood cells.
• Septic endocarditis.
___________________________
E. UNFRACTIONATED HEPARIN (Monomeric heparin) Enoxaparin, Fraxiparin
___________________________
F. Anti-Platelet, (Clopidogrel, Aspirin, Dipyridamol)
=======================
6. DIURETIC used in Heart Failure
Congestive heart failure (CHF)
Symptom for CHF is dyspnea, due to pulmonary edema 93% of patients, peripheral edema 70%.
As early as the 1600s, mercurial-based diuretics were used for the treatment of edema, termed dropsy. The 20th century saw the advent of carbonic anhydrase inhibitors, followed by thiazide diuretics, and finally loop diuretics.
Currently, Diuretics is an integral component to the treatment of acute and chronic heart failure, but mortality benefit or an alteration in disease progression depends on holistic treatment of CHF.
Guidelines are based on expert opinion.
Their effectiveness may be limited by adverse effects including electrolyte imbalances and neurohormonal activation.
Pharmacology
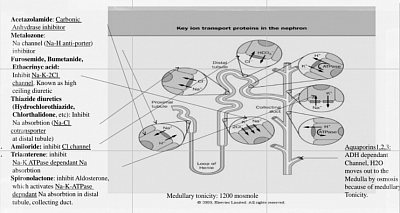
a) Loop diuretics are furosemide, torsemide, bumetanide, and ethacrynic acid. They inhibit the Na-K-2Cl cotransporter in the thick ascending limb (TAL) of the loop of Henle. TAL is water impermeable. By effectively inhibiting sodium reabsorption, they also reduce medullary Na concentration, thus reduce medullary Tonicity, thus reduce water absorption from Collecting tubule through Aquaporins. This causes high ceiling diuresis. The loop diuretics bind to the luminal surface of the transporter; thus, they must reach the tubular lumen by glomerular filtration.
Oral absorption of furosemide widely varies. Bumetanide and torsemide have greater bioavailability and more predictable pharmacokinetics.
All loop diuretics except ethacrynic acid contain a sulfonamide group. Individuals who are allergic to sulfonamide antibiotics may be given Ethacrynic acid.
b) Thiazide diuretics: commonly used for hypertension, and adjuncts in the management of heart failure. They inhibit the Na-Cl symporter in the distal convoluted tubule, leading to decreased sodium and water reabsorption.
Loop diuretics and thiazide, cause increased Na delivery to collecting tubule as they reduce Na absorption in their respective sites. Thus more Na is absorbed in collecting tubule in exchange of potassium and acids. This causes hypokalaemia and alkalosis.
c) Spironolactone inhibits the aldosterone receptor (MRA, mineralocorticoid receptor antagonist) in the cortical collecting duct, also limiting sodium (in exchange of potassium and bicarbonate, thus causing hyperkalaemia and acidosis as a complication), and water reabsorption. Its diuretic effect is relatively weak, and its onset of action is slow. But it reduces proteinuria (by modulating glomerular filtration pores), and reduces renal and cardiac fibrosis, thus known as renoprotective and cardioprotective. This is steroid, thus cause cushingoid picture and hyperkalemia. Another similar drug is Eplerenone. A new non-steroidal with less hyperkalaemia is Finerenone (approved for diabetic nephropathy).
Mechanism of action in CHF
The pulmonary and peripheral edema seen in CHF are the result of multiple physiologic disturbances. Decreased cardiac output leads to relative renal hypoperfusion that stimulates neurohormonal activation of the renin-angiotensin-aldosterone system (RAAS). Sodium and free water retention occur, resulting in an increase in both volume and pressure in capacitance vessels. Hydrostatic pressure elevation leads to fluid extravasation into peripheral tissues as well as the lungs.
The Frank-Starling law describes the mechanism whereby a normal heart under a physiologic range of filling pressures increases stroke volume proportionally with an increase in preload. In contrast, in acute decompensated heart failure, a myopathic heart subjected to very elevated filling pressures is not able to effectively increase stroke volume.
Acute elevation of left ventricular preload (end-diastolic pressure) directly leads to elevated left atrial pressures and pulmonary edema.
Diuretics reduce intravascular volume, leading to a decrease in central venous pressure, right and left heart filling pressures, and pulmonary vascular pressures. Venous capacitance increases, and intrapulmonary fluid returns to the circulation. The left ventricular volume is smaller, and cardiac output typically increases.
In the setting of mitral regurgitation, the reduced left ventricular volume improves mitral leaflet coaptation and decreases the regurgitant volume.
Diuretic resistance explains why some patients require high doses of diuretics or have a decreased response to diuretics over time. Several mechanisms contribute to diuretic resistance:
(1) The “braking phenomenon” refers to a short-term resistance following a bolus dose and may be related to neurohormonal activation that acts to preserve intravascular volume.
(2) A longer term resistance may be due to compensatory hypertrophy of the distal convoluted tubule, which avidly reabsorbs sodium and counteracts the natriuretic effects of loop diuretics.
(3) Additionally, as the GFR decreases in chronic kidney disease (CKD), a higher dose of diuretic is necessary to achieve therapeutic effect.
(4) Finally, GI absorption and subsequent bioavailability of oral diuretics may be impaired in heart failure due to bowel wall edema.
Outcomes & Complications
The efficacy of diuretics in improving symptoms of heart failure such as dyspnea and edema is desired and achieved.
a) However, studies have uncovered a correlation of diuretic dose with renal dysfunction, sudden death, hospital length of stay, and overall mortality.
b) Various electrolyte abnormalities can occur. Inhibition of the Na-K-2Cl channel leads to increased sodium delivery to the distal tubule and cortical collecting duct. Via the ENaC channel (Na-K antiporter), distal sodium is reabsorbed at the expense of potassium loss, resulting in hypokalemia.
c) Hypomagnesemia can occur as well, potentiating the hypokalemia. This imbalance may contribute to arrhythmias.
d) Chloride losses can lead to a hypochloremic metabolic alkalosis. Significant alkalosis can decrease respiratory drive in a patient with respiratory failure.
e) Hyponatremia can also occur with diuretics, more commonly with thiazide than loop diuretics, and especially if large amounts of free water are ingested. Although hyponatremia itself is rarely symptomatic, its occurrence is a marker of poor prognosis.
f) Diuretic use can lead to worsening renal function. Whether higher diuretic doses directly lead to the development of renal failure (cardio-renal syndrome) or are simply a marker for patients at risk is debatable. Newer data suggest that renal failure is more closely associated with elevation of central venous pressure than relative intravascular volume depletion from high dose diuretics.
g) Because diuretics acutely decrease left ventricular preload, they can lead to a reflex neurohormonal stimulation of the sympathetic nervous system (SNS) and renin-angiotensin-aldosterone axis. Numerous studies have determined that activation of these pathways contributes to the pathophysiology of heart failure, thus potentially undermining the benefits of diuretic use. This mechanism may also explain why various studies have failed to show a mortality benefit from diuretics use.
Concurrent treatment with neurohormonal blockade (ie, vasodilators, beta blockers, renin-angiotensin-aldosterone system antagonists) may improve outcomes, although this has not been systematically studied.
________________________
CLINICAL USE
Outpatient
A) daily frusemide. +/-potassium supplements.
B) Sodium restriction, less than 2 g daily, is essential.
C) 3 to 6 monthly vidit, eeight chsrt, electrlyte acid base monitoring
D) Frusemide serum half-life of only 1.5 hours, BD dosing.
E) to restrict dietary sodium intake and usage of nonsteroidal anti-inflammatory drugs (NSAIDs) must also be considered.
Inpatient
A) intravenous (IV) dose of a loop diuretic is typically given. The selected dose needs to be progressively higher as the GFR decreases.
B)The multicenter DOSE study explored the effects of high dose versus low dose diuretic use in acute decompensated heart failure. High-dose furosemide led to greater diuresis and improvement in overall symptoms compared to a low-dose regimen. Renal dysfunction was more common in the high-dose group, although at 60 days follow-up, creatinine levels were similar in both groups.
Continuous infusion may lead to an overall lower diuretic dose, limiting toxicity such as ototoxicity. The DOSE study was the most comprehensive trial to investigate the effects of continuous versus bolus diuretic dosing regimens in acute decompensated heart failure.
C) Combination:
i. Thiazide: (chlorothiazide, hydrochlorothiazide, and the thiazide-like diuretic metolazone). To overcome the resistance to loop diuretics associated with reactive hypertrophy of the distal convoluted tubule (DCT) of the nephron. By blocking the Na-Cl channel in the DCT, they hinder the avid sodium reabsorption that limits loop diuretic efficacy.
ii. For patients who have a significantly reduced GFR or diuretic resistance, sequential nephron blockade can be extended to include the proximal convoluted tubule, with acetazolamide, and the cortical collecting duct, with spironolactone.
iii. Potassium sparing diuretics
The aldosterone antagonists spironolactone and eplerenone have been shown to be effective therapies for chronic heart failure, based on the RALES and EPHESUS studies. Action is their inhibition of aldosterone rather than diuretic action. They may be beneficial in counteracting the hypokalemia from loop diuretics. They may also enhance diuresis in the resistant patient.
D) Non-diuretics
1) The vasopressin antagonist tolvaptan has been studied in heart failure. By inhibiting the vasopressin receptor in the distal nephron, it causes aquaresis. It both causes volume loss and combats the hyponatremia which is common in heart failure and a poor prognostic indicator.
The EVEREST trial studied short term use of tolvaptan versus placebo in addition to standard therapy (including diuretics) in acute heart failure. Serum sodium increased with tolvaptan. Patients had rapid improvement in symptoms, although no significant difference was noted by day 7 or discharge. The use of tolvaptan among the currently available treatments for acute heart failure needs to be determined.
2) ULTRAFILTRATION (Aquapheresis)
for reducing intravascular volume and managing pulmonary congestion. The UNLOAD study was a randomized controlled trial of UF versus intravenous diuretics in patients with acute decompensated heart failure. At 48 hours, greater weight loss noted with UF, dyspnea were similar, no difference was noted in rise in creatinine. Ninety-day hospital readmission was lower in patients who had received UF.
The following are contraindications to UF:
Evidence of hemodynamic instability
Acute coronary syndromes
Serum creatinine level of >3.0 mg/dL
Hematocrit of >45%