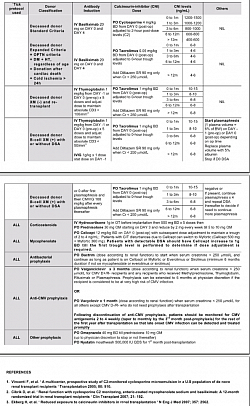
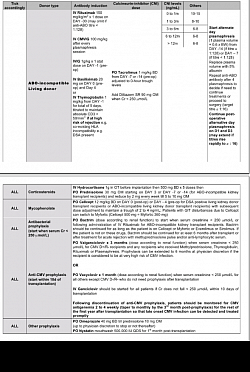
IMMUNOSUPPRESSION & TOLERANCE PARADIGM SHIFT PROTOCOLS
Immunosuppression in Kidney Transplant Programme and Paradigm shift to Tolerance
Contents
- Cellular and humoral mechanism
- Transplant tolerance
- Complete tolerance - congenital, acquired
- Prope tolerance
- CAR-T therapy for tolerance
- References
- TK TRANSPLANT Protocols
____________
1. Cellular and humoral mechanism in physiology, rejection and tolerance
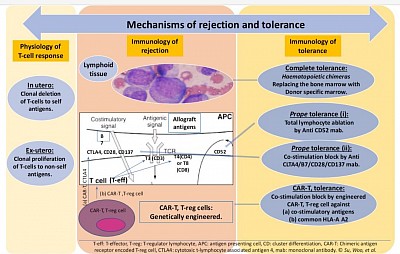
The figure explains basic immunological behavior of lymphoid tissue toward self and non-self antigens during embryogenesis and in adult life. It also shows the mechanism of graft rejection and strategies of graft tolerance.
_____________
2. Transplant tolerance
Transplant tolerance is defined as a state of donor-specific unresponsiveness to the allograft without a need for on going pharmacologic immunosuppression. These strategy could eliminate many of the adverse events associated with immunosuppressive medicines.
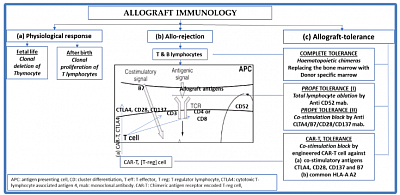
- In embryogenesis, mechanism of T-cell tolerance is the clonal deletion of auto-reactive T-cells in the thymus, haematopoietic and other lymphoid tissue, to the foetal antigens so that the organism is rendered self-tolerant to self-antigens. Therefore there is clonal expansion of T-cells, not exposed to exogenous antigens, and not reactive to the foetal antigens.
- In ex-utero life, the process of deletion is reversed to the state of proliferation on exposure to exogenous antigens whether organ or organism.
- Against organism, survival of the individual depends on antimicrobial agents and protection depends on vaccination.
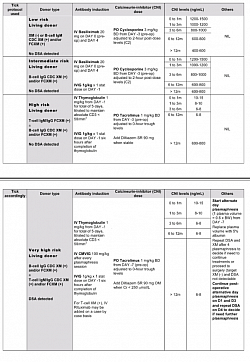
- For grafted organs, we need immunosuppression unless state of tolerance is achieved.
- Since, the whole process of immune identification and rejection is carried out by haematological immune cells, the principle guide to tolerance, the suppression of this mechanism is the hall mark of graft maintenance and possible tolerance.
- In theory and practice, there are three strategies of achieving tolerance:
- Complete tolerance: achieving a state of haemochimerism of recipients and donors lymphoid tissue.
- More recent, prope or near tolerance where immune-reactive t-cells are eliminated or inhibited.
- The most recent, CAR-T therapy using t-reg cells, engineered for ablating t-cell immune-reactivity through anergy.
_____________
3. Complete tolerance
- Congenital complete tolerance takes place in Transplant between monozygotic twins as there is no immune-reactivity between the twins because of genetic similarities (1).
- Acquired complete tolerance was achieved anecdotally in multiple myeloma cases where total bone marrow, and lymphoid ablation was obtained by whole body irradiation and chemotherapy, followed by donor specific bone marrow transfusion to repopulate the recipients marrow and lymphoid tissue before renal transplantation – a form of haematological chimerism (2). As the immunogenic t-cells belong to the renal donor, complete tolerance is achieved. Even though, not a reality in clinical transplantation yet, experimental animal transplantation is promising.
____________
4. Prope tolerance
Two types of prope or partial tolerance are currently known.
- i) Pioneered initially by Dr Calne with use of Campath 1H (Cambridge University Pathology) in clinical organ transplantation. It showed achieving a partial tolerance where allografts could be maintained with minimal immunosuppression. Campath 1H is a humanised chimeric rabbit monoclonal antibody against human CD52 lymphocyte receptor, where Fc segment of rabbit IgG monoclonal antibody is replaced by human Fc segment. CD52 antigen is present in all lymphocytes. Thus administration of campath causes lymphocyte depletion for long time. Later new lymphocytes, generated from lymphoid tissue, behave like niave lymphocytes to the grafted organ. As such, do not show marked rejection response to the foreign organ. Two IV administration of 20 mg at 3 months interval, achieves ablation of lymphocytes for long time, allowing the recipients lymphoid tissue to repopulate with lymphocytes, naïve to the allograft. Thus achieving immune tolerance. INTAC, 3C and similar studies showed promising results for minimising long-term immunosuppression (3).
- ii) More recently with newer unique mab against co-stimulatory signal inducing TCR (CTLA-4, CD28, B7, CD137), t-cell co-stimulation (signal-2) is blocked inducing t-cell anergy.
BENEFIT study used Belatacept, a selective co-stimulation blocker which is a human fusion protein combining extracellular portion of cytotoxic t-lymphocyte associated antigen 4 (CTLA-4) with Fc of human IgG1, showed prope tolerance in phase 3 trial (4).
_____________
5. CAR-T therapy
Principles of CAR-T therapy:
1)Anti cancer therapy: Immune cells (T-regulator cells) from patient’s blood (i.e. Acute lymphobkastic leukemia) is drawn and equipped with a Chimeric Antigen Receptor (CAR-T). The antigen receptor is a genetically engineered receptor (mostly a monoclonal antibody against the antigen. The antigen is the target cell surface protein i.e. lymphoblastic cell surface antigen). The mRNA producing the mab is synthesized in laboratory. It is incorporated in a harmless virus used as vector. The virus containing the genetic sequence is then transfected in patient's T-reg cell. Thus T-reg cell is converted into a CAR-T cells. These cells are multiplied to a million count, and then transfused back to the patients blood stream. The CAR-T cells containng the mab get attached to the surface antigen of i.e. ALL cells, and destroy the cancer cells. The receptor binds itself to a specific protein on the cancer cell and activates the CAR-T cells to kill the cancer cells.
2) CAR-T therapy in transplant tolerance using Chimeric Antigen Receptor for regulatory T-cells (T-reg): When you can block Signal-2, there is T-cells anergy, and fail to induce graft rejection.
Signal-2 is known as Co-stimulatory signal: APC surface antigen B7-1 & 7-2 get attached to TCR CD28 (homologous to CTLA-4), of T-cell receptor. Upon this signal-2 activation, the lymphocyte, which could be either T-4 or T-8 cell, is activated when CD3 of the T-cells is activated by corresponding antigen of APC. The T-cell proliferation occurs, to cause graft rejection. Therefore, blocking signal-2 either by monoclonal antibody against CTLA-4 antigen or APC B-7 antigen, T-cell activation can be blocked and you get tolerance by so called T-cells anergy.
BENEFIT study used Belatecept (mab against B-7) for this purpose.
This mab can be delivered by CAR-T cell to the target cell antigen CTLA-4 on the surface of T-cells to block attachments with B-7 antigen of APC instead of iv Monoclonal antibody (Belatecept). Thus produce same kind of T-cell anergy producing tolerance. This is explained below:
CAR-T (virus containing the genetically engineered mab forming mRNA against CTLA-4 antigen, is transfected to patients isolated T-reg cells), multiplied and transfused back to patient during transplantation. This CAR-T cells containing the mab against the CTLA-4, find the T-cells containing CTLA-4 antigen on their surface and blocks it. Thus APC containing the graft antigen with their B7-1 & 7-2 surface antigen can not bind to the CTLA-4 antigens as they are already occupied by mab from the CAR-T. Thus, co-stimulation as described above is blocked and T-cells activation and proliferation does not occur, resulting in tolerance.
Most recently, this research on CAR-T therapy targeting T-reg (regulatory T-cells) has been pioneered. The genetically engineered T-reg cells are aimed to block co-stimulatory signaling receptors as mentioned above. Thus blocks T-effector cell up-regulation. The readministered genetically engineered CAR-T-reg cells with receptor (mab) component for CTLA-4 antigen of T-cell as described above, blocks CTLA-4, making them unavailable for binding to B7 antigen of APC, resulting in absent co-stimulation, and thus induce T-cell anergy to CD-3, CD-4 and CD-8 lymphocytes. This is akin to induction of tolerance in organ transplant. This has been shown to be effective in skin, islet cell graft and has been speculated to be effective in inducing tolerance in renal and liver allograft (5).
_____________
6. References
- Brown JB. Homografting of skin: with report of success in identical twins. Surgery. 1937. 102:558.
- Bühler LH, Spitzer TR, Sykes M, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002 Nov 27. 74(10):1405-9.
- Michael J. Hanaway, M.D, E. Steve Woodle, M.D., Shamkant Mulgaonkar, et al. Alemtuzumab Induction in Renal Transplantation. For the INTAC Study Group. N Engl J Med 2011; 364:1909-1919, DOI: 10.1056/NEJMoa1009546.
- F. Vincenti, B. Charpentier , Y. Vanrenterghem, et al. A Phase III Study of Belatacept‐based Immunosuppression Regimens versus Cyclosporine in Renal Transplant Recipients (BENEFIT Study). https://doi.org/10.1111/j.1600-6143.2009.03005.x (accessed on 13 January 2019)
- Qunfang Zhang, Weihui Lu, Chun-Ling Liang, et al. Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance. Front. Immunol. 9:2359. doi: 10.3389/fimmu.2018.02359.
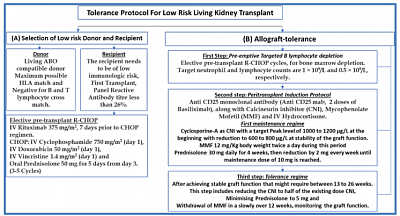
- Suhail SM, Woo KT (2020) Paradigm Shift to Tolerance from Conventional Immunosuppression in Renal Transplant: A Basic Strategy through Lymphocyte Depletion. J Nephrol Ren Dis 4:121.
- Suhail SM. Tolerance protocol of low risk living related transplant for developing countries. World J of Transplantation, May 2022.
____________________________